News

New distribution partnership in Europe
Press release: We are excited to announce that we are extending our existing partnership for the distribution of Benzalkonium Chloride with Actylis to also cover Germany, UK, Spain and Portugal.
Read more
We are attending BPI Europe 2024
We are pleased to announce our attendance at the upcoming BPI Europe congress, taking place April 9-12 in Vienna. Make sure you join us at booth 240.
Read more
We are presenting at World Vaccine Congress
Join us at the most important vaccine event of the year - the World Vaccine Congress in Washington on April 2-4! Our talk, "Improving cell proliferation and productivity through Insulin supplementation in animal-free cell culture media," is not to be missed!
Read more
Novo Nordisk Pharmatech Annual Report 2023 video
Hear CEO Ulla Grove Krogsgaard Thomsen talk about the highlights from 2023 and get a glimpse of the outlook for 2024.
Read more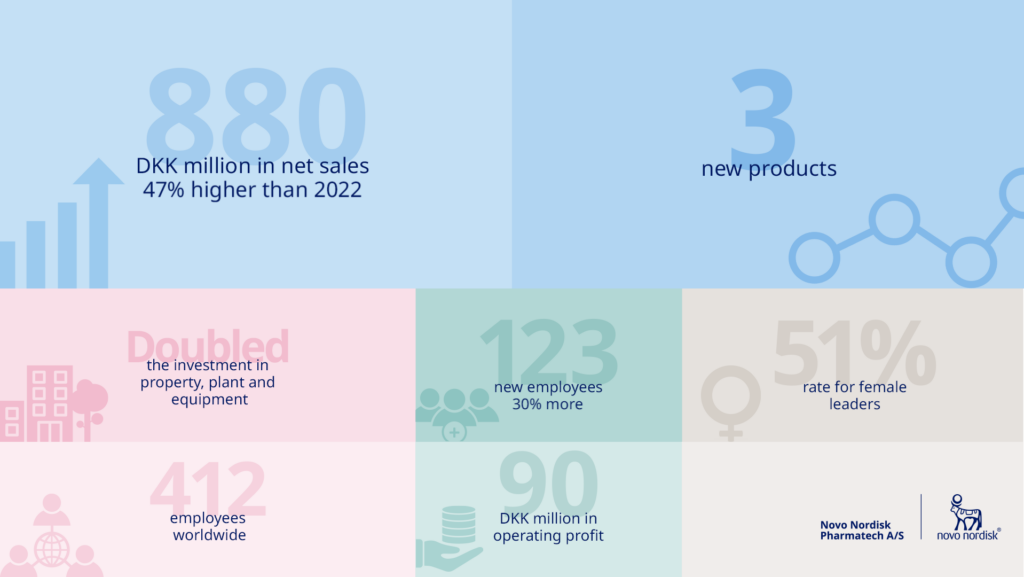
Novo Nordisk Pharmatech Annual Report 2023
In many ways, 2023 has been a transformational year where we made significant progress on our Novo Nordisk Pharmatech growth strategy.
Read more
Novo Nordisk Pharmatech at BioProcess International in Boston
As a leading provider of Recombinant Insulin, we are thrilled to showcase our products at the largest bioprocessing event and meet with industry professionals.
Read more
Novo Nordisk Pharmatech at BIO Asia Taiwan
We're excited to announce our presence at this year's BIO Asia Taiwan event, where you can meet us at booth L724 on July 27-30. If you are working within the topical segment, you could also benefit from attending our ‘Why choose a non-alcohol based antimicrobial for topicals’ presentation on the stage.
Read more
Antiseptic for wound care
Which antiseptic should I use for wound care? If you plan to use an antimicrobial antiseptic in a wound care product or device, you should download this free white paper.
Read more
Celebrating a day of inspiration and empowerment
We are dedicated to making a difference in our society and empowering those seeking employment. Therefore, we have recently hosted a inspiration day to provide guidance, support, and inspiration from unemployed people.
Read more
A day of inspiration, knowledge sharing, and fun
On Wednesday employees from Novo Nordisk Pharmatech met for an all-day event to deep dive into our strategy, our products, our customers, and the patients for whom this ultimately benefits.
Read more
New whitepaper for Ophthalmic and Nasal
If you are formulating multi-dose products for ocular or nasal use, you may face several challenges when selecting a preservative system. Here we explore and discuss these challenges and compare several antimicrobial systems.
Read more
Nasal delivery is very effective
Could nasal delivery be the route of administration for your new drug? This route is not only reserved for decongestants or anti-inflammatory compounds. Nasal delivery is a very effective and non-invasive route to administer drugs.
Read more
On-demand webinar AAV and LV
Do you want to increase viral vector productivity? We can show you how using Recombinant Insulin can significantly enhance the productivity of both, AAV and LV viral vectors in HEK 293 cells.
Read more
On-demand webinar BKC
It only takes 15 minutes to understand the importance of choosing the right antimicrobial! You will learn the importance of choosing the right antimicrobial as an excipient or active pharmaceutical ingredient.
Read more
Free Recombinant Insulin e-book
Understanding WHY insulin is a key ingredient in cell culture will help you identify HOW you can easily boost the performance of your biomanufacturing. This e-book will explain how insulin is enhancing the manufacturing of drugs.
Read more
Meet us at BPI Europe
We are excited to announce our presence at Bioprocess International Europe. You can visit us at booth 33 from May 9-11. As a leading provider of Recombinant Insulin, we are thrilled to showcase our products.
Read more
Stay surveys and interviews
We stand united. It is the foundation of our corporate culture and since our people are crucial for our successful journey, we have kick-started a new initiative to ensure our employees feel valued and developed. We have established an employee group with the purpose of making concrete initiatives based on employee feedback
Read more
Safety and Health at Work Day
Today is `Safety and Health at Work Day´ and this means a lot more to us than preventing something from going wrong. We also strongly focus on maintaining our culture as a fast-growing company. Our Senior Health & Safety Professional Graham Stenning is impressed by our ability to retain our corporate culture.
Read more
Carpooling with FDM
At Novo Nordisk Pharmatech, we are using the Danish carpooling app “Ta’Med” in collaboration with FDM. The purpose is to reduce CO2 emissions and at the same time strengthen our psychosocial environment – a win/win situation. Our experience so far is that carpooling brings us closer together across the value chain.
Read more
Case study about influenza virus
To improve the biomanufacturing in mammalian cells, we tested our recombinant insulin in the cell culture media of HEK293 cells producing influenza virus. We found that adding insulin to the feeding media of HEK293 cells boosts the production of the influenza virus up to 2-fold, compared to the media without insulin.
Read more
We expand the production
To support future growth and production expansions, we have invested in a new plot of land close to headquarters in Køge Nord (North). With the purchase, the needed space for future expansions is ensured while maintaining the synergies with the nearby headquarters and the interaction with local authorities.
Read more
Application landscape of Recombinant Insulin
From the production of monoclonal antibodies to cell and gene therapies, and vaccines, our recombinant insulin contributes to improve the lives of people living with arthritis, cancer, multiple sclerosis, and many more medical conditions.
Read more
New member of Board of Directors
The newest member of our Board of Directors is Julie Aunbirk. Julie has been with NNE for more than 23 years and has been heading up NNE’s largest area, covering all Engineering disciplines within Process & Facility Design.
Read more
Gain valuable hands-on work experience
Do you wish to kick-start your career by working closely with experienced colleagues while gaining valuable hands-on work experience? And doing so within all areas of the supply chain, from R&D and Production to Sales and Marketing?
Read more
This week is World Glaucoma Week
At Novo Nordisk Pharmatech, we are proud to be part of the journey to providing better glaucoma medicines. Our Benzalkonium Chloride products are used as an excipient to preserve ophthalmic solutions, gels, and ointments used to treat glaucoma.
Read more
International Women´s Day
Yesterday we celebrated International Women´s Day with an inspirational afternoon with our CEO, Ulla Grove Krogsgaard Thomsen, and our CCO, Samia Kappe. Two out of many strong female profiles at our company. "By sharing my story, I wish to inspire and support women in pursuing their carrier ambitions."
Read more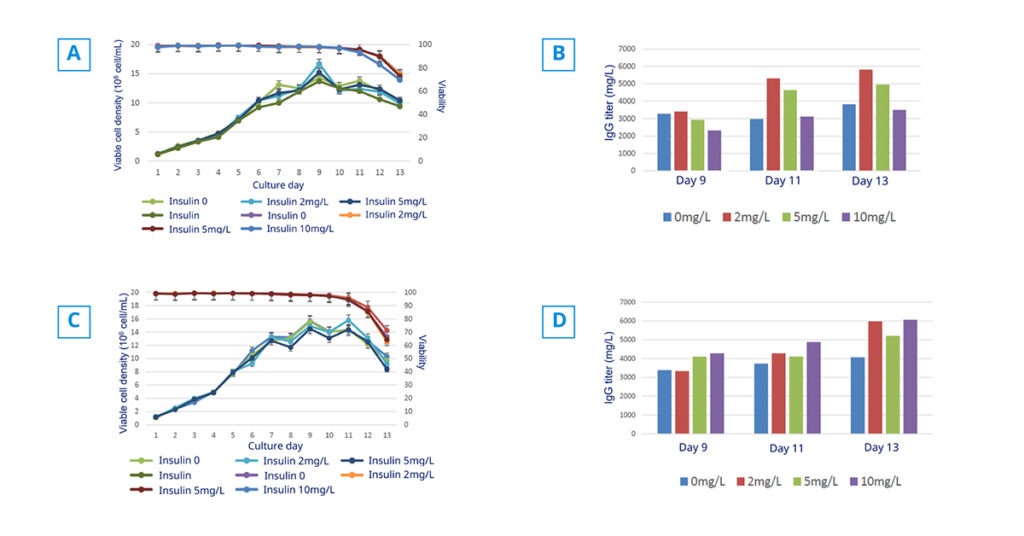
Case study CHO-K1
Are you looking for a method to increase the expression of recombinant proteins in Chinese Hamster Ovary (CHO) cells? In this study, we show the effects of animal-free recombinant insulin supplementation on CHO-K1 cell proliferation and productivity of IgG protein in fed-batch culture.
Read more
Key ingredient for biomanufacturing
If your goal in cell culture media optimization is to achieve high-performance and cost-effective processes, don’t compromise on the quality of its ingredients. Choose the certified quality of Novo Nordisk Pharmatech recombinant insulin, rely on its highest purity, and feel safe with its batch-to-batch consistency.
Read more
A look into manufacturing
Manufactured by Novo Nordisk in cGMP facilities, our highest-quality recombinant insulin is packed in our cGMP facilities and shipped worldwide in validated containers that preserve its quality over time. Are you curious to see how we take good care of the quality of your cell culture media ingredients?
Read more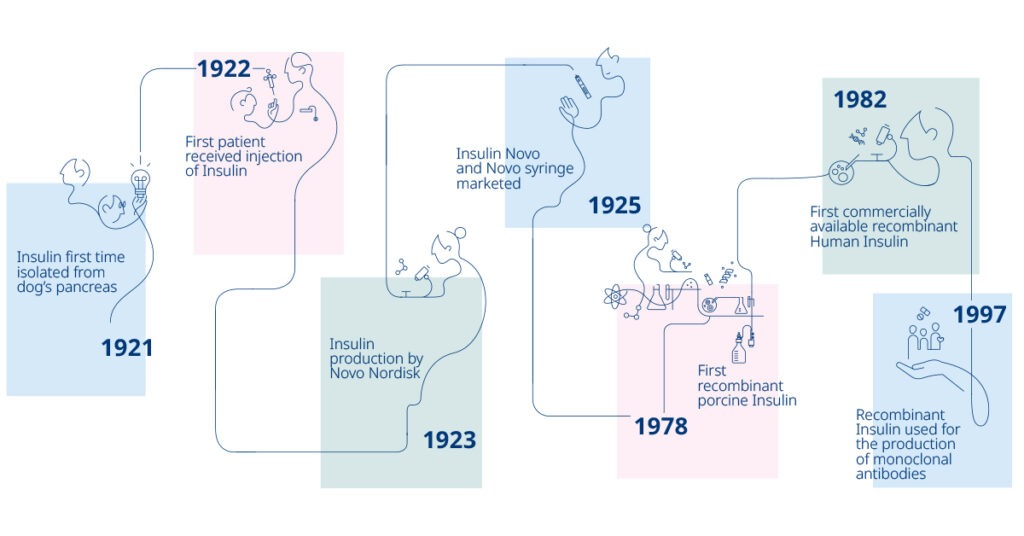
100 years of Novo Nordisk insulin
Novo Nordisk Pharmatech celebrates 100 years of Novo Nordisk insulin, sharing the excitement of being part of a historical company that has driven medical changes since 1923. Since then, significant improvements in how insulin is manufactured and administered to patients have made diabetes treatment safer and simpler.
Read more
Sustainability and impurities matters more than ever
Sustainability and impurities. Over 1000 industry leaders and experts were gathered last week at the #2023ACI Annual Convention, where impurities and sustainability were recurring topics. Both areas are close to our hearts when it comes to pharma-grade Benzalkonium Chloride for use in topical antiseptics.
Read more
Second leading cause of death
Novo Nordisk Pharmatech A/S is honored to contribute to the development and manufacturing of many available anti-cancer monoclonal antibody drugs, supplying Recombinant Insulin as a cell culture ingredient to boost protein productivity in cell culture.
Read more
We will plant 12.400 square meters of trees
Together with Klimaskovfonden, we will plant 12.400 square meters of trees in "Landsbyskoven i Ishøj." The trees will absorb 400 tons of CO2 over a 100-years period and contribute to the Danish CO2 reduction target. They will be a supplement to our existing initiatives in relation to reducing our climate and environmental footprint.
Read more
An easy drug product registration process
Do you want to ensure an easy drug product registration process? And save time and headaches and have peace of mind during your drug product approval? Our team of experts can support your regulatory needs and ensure a smooth registration process, whether your medicine is manufactured for your local or export markets.
Read more
We never compromise on quality
We never compromise on quality! We always strive to serve our customers through high product quality, extensive manufacturing and quality control, regulatory documentation, and a comprehensive risk mitigation strategy. That defines us, and we are not willing to ditch it.
Read more
We are expanding
We are expanding our business fast to meet the biopharma market demands. This past year, we went from 240 employees to 300 employees. Novo Nordisk has incredible and highly engaged employees, and we were recently awarded the Best Place to Work worldwide. No need to say that we are very proud of the acknowledgment.
Read more
Winter is here, Christmas is coming closer
Winter is here, Christmas is coming closer, and our Sales & Marketing team wishes you all a safe and relaxing holiday season. Last week the members of our Sales & Marketing department gathered in Denmark to reflect on the previous year and align on the plans for the new year.
Read more
CPHI India in New Dehli opened yesterday
CPHI India in New Dehli opened yesterday, and our Sales Manager for Europe and India can be found at booth 8 Q02 together with our partner Signet Excipients Pvt. Ltd., India´s largest distributor of pharmaceutical excipients. Together, we will share information about our pharmaceutical-grade Quats products.
Read more
Quats explainer video
Benzalkonium Chloride, Cetrimide, and Cetrimonium Bromide - also known as Quats - are powerful antimicrobial surfactants. They act against bacteria, fungi, and viruses by penetrating and destabilising their membranes, leading to their death.
Read more
Presentation at Cell UK
"Improving cell proliferation and productivity through Insulin supplementation in animal-free cell culture media" was part of the agenda at Cell UK in London this week, presented by our Global Product Manager Sara Bursomanno.
Read more
CPHI Worldwide opened today
CPHI Worldwide in Frankfurt opened today and if you wish to know more about pharmaceutical grade Benzalkonium Chloride, CTAB, or Cetrimide, then come by booth 110G02. We would love to meet both known and future customers the next couple of days.
Read more
Biologic World in Taiwan
Come and visit us and learn more about Recombinant Insulin as a key component in serum-free cell culture media for the production of therapeutic proteins. At booth 7 you will meet our Head of Sales Timur Özbay and our International Sales Manager Kerina Soh Sze Imm.
Read more
The “Ta’Med” Campaign
As part of our ongoing focus on sustainability, we now encourage our employees to participate in a carpooling campaign. By carpooling, our employees support more sustainable transport by reducing CO2 emissions.
Read more
Case study HEK293 cells
We have explored the effects of recombinant insulin on viral vectors production in HEK293 cells. The results are presented in a case story, and you can read the conclusion here.
Read more
Safeguarding your regulatory compliance
Our one-stop regulatory support provides you with everything you need in a fast and convenient way and our expert team is here to take the complexity and risk out of regulatory compliance for your whole product lifecycle
Read more
Are you attending BioProcess International in Boston?
Are you attending BioProcess International in Boston, September 27-30? And do you want to know more about Recombinant Insulin as a key component in serum-free growth media for cell culture media or for therapeutic proteins?
Read more
40 years in the same company is nothing short of impressive
Anette started in Nordisk Gentofte (later Novo Nordisk) back in 1982 in the microbial laboratory. As a newly qualified pharmacologist, her first tasks were sterile testing in what used to be a small department.
Read more
Cell culture in bioprocessing
Cell culture remains one of the most important bioprocessing tools we have today. The classic example of this process is the cell culture of a Chinese hamster ovary (CHO) cell line, used to express therapeutic proteins such as antibodies, cytokines, enzymes, and hormones.
Read more
Case study: Optimize CHO-S cell culture
This study focuses on the improvement of CHO-S cell growth producing the antibody Herceptin by addition of animal-free recombinant insulin (r-insulin) as a supplement to commercially available chemically defined media.
Read more
Located directly by a Natura 2000 area
It is well-known that beautiful surroundings give peace of mind. Therefore, we could not be more pleased about our location by Køge bay. We are located directly by a Natura 2000 area consisting of a barrier beach, a lagoon, and on the land side of it, salt marshes.
Read more
Our most valuable resource
With an average score of 89 about our people (on a scale from 0 to 100), we are very proud of the result and will do our best to keep our customers satisfied – both at our headquarter in Denmark and at our affiliates in Singapore and the US.
Read more
Enabling the next wave of innovative drug therapies with speciality enzymes
In the evolving biotechnology industry, enzymes are important process aids. “Specialty enzymes are proteins that can act as very specific biocatalysts to accelerate reactions and produce the desired target molecule in pharmaceuticals,” explains Kristoffer Laursen.
Read more
"Kemien skal være i orden"
"Dansk Erhverv Magasinet" interviewed Ulla Grove Krogsgaard Thomsen about her background at Novo Nordisk, her first years as a leader in the then male-dominated world, and how she strives as the CEO at Novo Nordisk Pharmatech. “To be successful as a leader, it is not enough to have control of the chemical processes”
Read more
Audit of ISO standard certificates
In May, we had the yearly following-up audit of our 3 ISO standard certificates, and we are proud to say there were no findings. This is a result founded by dedicated work among our employees complemented by a strengthened learning culture across our whole value chain at Novo Nordisk Pharmatech
Read more
BPI Europe 2022
On May 18-19 our team will attend the conference and staff our booth in the exhibition hall. In addition to learning more about our existing and future products, you even have the chance to win great prizes on our shuffleboard. Stop by and say hello at booth 71.
Read more
Our culture is an essential part of our business framework
Making sure our company is a great place to work ensures that we are not just a good company but a great one. This mindset made us close the entire company for a day to discuss our culture. Focusing on what makes us strong is especially important now as the number of employees grows every month.
Read more
US Sales Managers on the road
In May you have an excellent opportunity to meet our two US Sales Managers on the road. Mike Vincent and Pari-Naz Javaheri will attend three conferences and trade shows: Prep Symposium in Baltimore, CPhI North America in Pennsylvania and Interphex in New York City.
Read more
A kestrel has moved in
We have expanded our facilities and welcomed a new member of the Novo Nordisk Pharmatech family: a kestrel. The kestrel has been a regular visitor to our site since last year. And now we have installed a nest box for him and his companion. Supporting our Circular for Zero strategy, the 8-meter high pole has been recycled.
Read more
Our growth strategy
In 2022 we are executing on our growth strategy, preparing for the launch of our first recombinant GMP grade enzymes. This supports our mission to enable better medicines in the biopharmaceutical and Advanced Therapy Medicinal Products markets. The expanded global presence achieved with the establishment of the sales office in the USA will enhance and support our future external product launches.
Read more
Annual report 2021
Novo Nordisk Pharmatech delivered a satisfactory 2021 result with external sales above our expectations, a net profit that was on par with last year, solid progress within our production footprint, and our pipeline of new products. You can read the full annual report for 2021 here.
Read more
Key component in serum-free growth media for mammalian cells
Recombinant insulin is a key component in serum-free growth media for mammalian cells. It is used many cell lines, such as CHO-s, HEK 293, and Sf9, for the manufacturing of monoclonal antibodies, virus vaccines and gene therapy products. It can also be used in other biological drug products.
Read more
Meet our Customer Support team
They take good care of our customer’s purchasing orders and are responsible for ensuring that orders arrive timely at our customer’s facilities worldwide. Besides order handling and shipping, our customers also communicate with Customer Support if they have any requests for quality documentation.
Read more
Life science executive of the year
We are proud to announce that our CEO Ulla Grove Krogsgaard Thomsen has been nominated as Life science executive of the year. One of the reasons for the nomination is that she appears with great authenticity in her persona and leadership and has managed to set clear directions, purposes, and priorities for her organizations.
Read more
How do Quats work?
Quaternary ammonium compounds, commonly known as Quats, are a group of cationic surfactants with a high level of antibacterial activity and an important role to play in the pharmaceutical industry. Key examples include Benzalkonium Chloride (BCK), Cetrimide, and Cetrimonium Bromide (CTAB), but how do they work?
Read more
Novo Nations at Pharmatech
As a global company, we hire people from all over the world and focus on creating an inclusive atmosphere. We are proud of having 14 different nationalities among our 200+ employees on-site at Køge, Denmark. Initiated by some of our international colleagues, we launched Novo Nations at Pharmatech last week.
Read more
Wide range of applications
Proven under ICH Q1 conditions, our Quats are very stable. They are powerful antimicrobial surfactants, soluble in aqueous and oily phases, and basically active at all pH ranges. They can be used for many applications requiring high purity and quality, such as ophthalmics, nasal sprays, topical products, vaccine, and gene therapy processes.
Read more
The purpose of an excipient
Our Quats products, e.g. Benzalkonium Chloride (BKC), are safe and effective pharmaceutical excipients. But what is the purpose of an excipient, and what is the difference between an excipient and an active substance?
Read more
As promised, trees will be planted!
Before Christmas, we decided to collect comments on a LinkedIn post. Every comment would result in a planted tree together with One Tree Planted. We chose to expand and plant 100 trees on the continents possible = 600 trees planted.
Read more
We will plant a tree for your opinion!
Since August, this has been our procedure whenever we have sent our NPS survey to our customers, and we have donated a tree via One Tree Planted for every NPS score we have received.
Read more
Managing risk is central to the business in Novo Nordisk Pharmatech
Managing risk is central to the business in Novo Nordisk Pharmatech A/S, as it is critical for us to protect our assets, our employees, and the business of our customers.
Read more
Novozymes and Novo Nordisk Pharmatech announce collaboration agreement on specialty enzymes
Novozymes and Novo Nordisk Pharmatech announce a collaboration agreement that combines the companies’ joint competencies and interests within the development of specialty enzymes for use in biopharma processing and regenerative medicines. An initial product is expected to be brought to commercialization within a few years.
Read more
Webinar with Helm de Mexico
In a constantly moving market for antimicrobials of high quality, we always support our distributors in their strategy to educate the local markets. On November 25, Helm de Mexico hosted a webinar: “Sourcing the Best Quality, GMP Antimicrobials - Quats from Novo Nordisk Pharmatech”.
Read more
Our cGMP Benzalkonium Chloride
Quaternary ammonium compounds (Quats) such as Benzalkonium Chloride are well-known antiseptics, effective against bacteria, fungi (yeast and mold) and algae. 𝘽𝙪𝙩 𝙬𝙝𝙖𝙩 𝙖𝙗𝙤𝙪𝙩 𝙫𝙞𝙧𝙪𝙨𝙚𝙨?
Read more
CPhI Worldwide Expo in Milan
On November 9-11, we were an exhibitor at the physical CPhI Worldwide Expo in Milan in the Active Pharmaceutical Ingredients (API) zone. We exhibited from booth 18K49 in Hall 18 at the Fiera Milano exhibition centre and talked about our world-leading range of pharmaceutical grade Quats at the booth.
Read more
Novo Nordisk Pharmatech at Exhibitions
During week 43, we had the pleasure of re-connecting and networking with the market in person. Both at Cell UK in London, World Bioprocessing Summit in Berlin and Antibody-based Therapeutics and BioProduction Forum in Shanghai.
Read more
From cultured meat to artificial blood
Recombinant Insulin has for decades protected the lives of millions of diabetic patients worldwide. Meanwhile, Insulin has become an instrumental component in serum-free growth media for mammalian cells, helping scientists manufacture innovative drug products.
Read more
Ensuring quality assurance
In Pharma Focus Asia newest issue, you can read the interview with our Managing Director Ulla Grove Krogsgaard Thomsen. In the interview, she talks about the role of formal documentation in quality assurance, ensuring the quality of materials and products and the benefits of using cGMP Quats as excipients.
Read more
Flower meadow and biodiversity
To increase biodiversity through better habitats and food for insects, birds and other animals, we have established a flower meadow on our production site in Køge. We have established the flower meadow on a 1,500 m2 area reserved for future expansion of production.
Read more
Distribution agreement with HELM de México
Novo Nordisk Pharmatech has entered into an agreement with HELM de México to distribute pharmaceutical grade quaternary ammonium compounds (Quats). HELM de México, S.A. has marketed raw materials for over 120 years with more than 100 subsidiaries, sales offices and participations in over 30 countries.
Read more
From student intern to a full-time employee
The road from being a student intern or assistant to a full-time employee is not long at Novo Nordisk Pharmatech A/S. As part of our talent pipeline, we can sometimes offer full-time positions within their field upon completion of their studies. Meet two of our recent hirings, Emma Lillebro Striib Nielsen and Dimitri Kobrossi.
Read more
We are really happy for the support you have given us
At Novo Nordisk Pharmatech A/S, we work with the “triple bottom line” – covering our environmental performance as well as our financial and social performance. We value the relationship to the surrounding society and therefore support local activities, events and projects within healthy lifestyle promotion.
Read more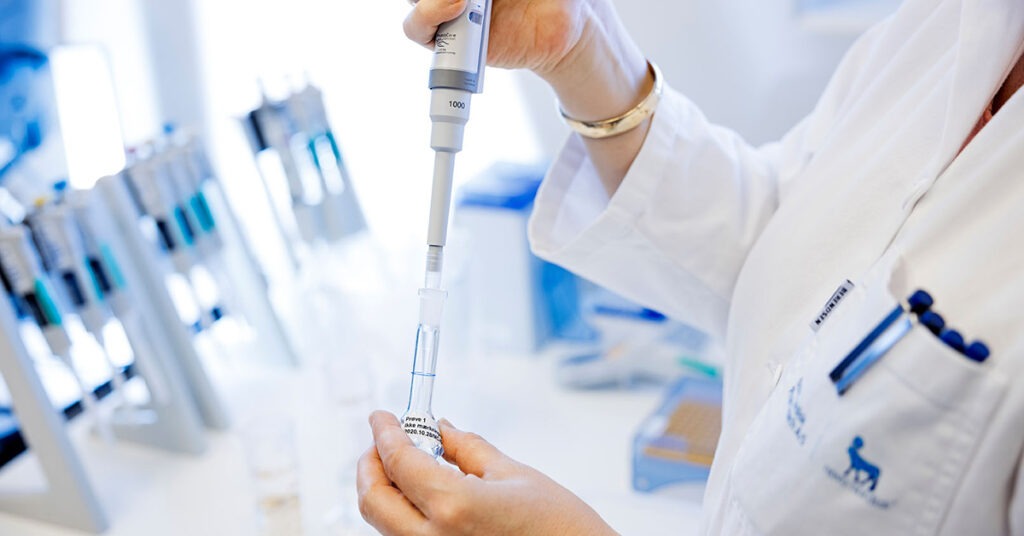
Using recombinant insulin in DoE for media development
In the presentation “Why Use Recombinant Insulin in DoE For Your Media Development?”, Product Manager Chantale Julien share a case study conducted in partnership with Universitat Autònoma de Barcelona.
Read more
New Sales Managers in Boston
Meet our Sales Managers at our newly established sales office in Boston. They have been on site in Denmark the previous weeks to learn about our business, existing and future products, our markets and strategies.
Read more
CO2 neutral company since 2018
Since 2018, Novo Nordisk Pharmatech is considered a CO2 neutral company. This environmental improvement is due to an agreement from 2015 on energy distribution, ensuring that Novo Nordisk Pharmatech is only supplied with renewable energy produced by windmills.
Read more
Our yearly NPS score in 2020 was 83
"Everyone is efficient, helpful and responsive to our questions and requests. We are appreciative of all they do to make our experience so positive. The product is the primary reason we sought out the relationship, and the service is why we seek to keep it as our primary source."
Read more
I have grown in my professional identity
"For the last five months I have been a part of the People & Organisation unit as an intern. I have grown in my professional identity as organisational psychologist while at the same contributing to creating meaningful change at Novo Nordisk Pharmatech."
Read more
Distribution agreement with IMCoPharma a.s.
Novo Nordisk Pharmatech A/S has entered into an agreement with IMCoPharma a.s. for the distribution of their pharmaceutical range of quaternary ammonium compounds (Quats) to the healthcare industry in Russian market and CIS countries.
Read more
Fostering an inclusive environment
Among our leaders and in our departments, we have initiated dialogues around fostering an inclusive environment during June. Through testimonies and examples, we have investigated our own biases and pitfalls, and through conversations in the teams, our leaders have asked for feedback on how they can be more inclusive as leaders.
Read more
Meet Verena Reiser
Meet Verena Reiser. Verena is an enzyme specialist in our Research & Development department and has been a part of Novo Nordisk Pharmatech A/S since 2019. Verena is part of the core behind our 2025 strategy for enzymes and is working on creating the framework for our future enzyme development.
Read more
Jacob Petersen - newest member of our Board of Directors
We are pleased to welcome Jacob Petersen as the newest member of our Board of Directors. Jacob has since 2018 been head of Stem Cell R&D Unit at Novo Nordisk, being responsible for the entire R&D value chain. He has also been involved in promoting a more international presence for Novo Nordisk Research.
Read more
Virtual Distributor Event
May 27-28 2021, we ran our first virtual Distributor event. With more than 90 participants from our global Distributor network we were excited to broadcast live from our site in Køge (Denmark) and it turned out to be a success.
Read more
Get to know Novo Nordisk Pharmatech
You can visit our site in Køge (Denmark) and get more knowledge about the company in our corporate video. At Novo Nordisk Pharmatech A/S we provide pharma grade Quats and Insulin Human AF to some of the world´s largest pharmaceutical and biopharmaceutical companies. All manufactured at cGMP facilities
Read more
What is Recombinant Insulin? See our FAQ
What is Insulin Human AF? Which applications is our Insulin Human AF suitable for? And what insulin concentration do we recommend adding to the base medium to improve growth or expression when using CHO (HEK293, BKH…) for protein expression?
Read more
Meet Henriette Lentz
Henriette is the Associate Manager for our EHS (Environment, Health and Safety) team and is responsible for health, safety and risk issues. At Novo Nordisk Pharmatech we have been proudly CO2 neutral since 2018. We are supplied with renewable energy produced by windmills, while our gas consumption comes from biogas certificates.
Read more
Meet Maria Bukh Almstrup
Maria is P&O Senior Manager for our HR, Communication and Training department. At Novo Nordisk Pharmatech we are an international organisation with more than 14 different nationalities. We cover the entire value chain and we are very dependent on each other.
Read more
Get answers to your questions - FAQ
How do we ensure the quality, purity and consistency of our Quats products? What is the difference between Cetrimide and CTAB (Cetrimonium Bromide)? And do we keep stock for all Quats products?
Read more
Meet Maxim Sierra Koltomov
Maxim is the Associate Manager at our Supply Chain team. The team focuses on sourcing raw materials, executing the production plan and supporting our future product pipeline.
Read more
Novo Nordisk Pharmatech´ s new CEO has a head start
Ulla Grove Krogsgaard Thomsen, the new CEO at Novo Nordisk Pharmatech A/S, comes with a strong background in Research & Development and Product Supply within Novo Nordisk A/S. Over the last two years, she has been a board member at Novo Nordisk Pharmatech, which has now appointed her as their new CEO.
Read more
Meet Tine Holland Frimann
Meet Tine Holland Frimann. Tine is GMP Area Specialist in our Quality & Regulatory affairs department. The department covers quality control, quality assurance and regulatory affairs.
Read more
2021: New sales office and multipurpose facility
In 2021, we will prepare the launch our first recombinant GMP grade enzyme aimed at the biopharmaceutical and regenerative medicine market. In order to support the launch and subsequent product launches, we are establishing a sales office in the US.
Read more
Meet Mikkel Mølvig Dalsø
Meet Mikkel Mølvig Dalsø, the Associate Manager in our Manufacturing. Here we we produce pharma grade quaternary ammonium compounds, also known as Quats.
Read more
Annual report 2020; Letter from management
2020 has been a year where no part of society has been left untouched due to COVID-19. This also goes for Novo Nordisk Pharmatech, who for better and worse, has been able to navigate this challenging business environment.
Read more
Meet Kate Lützhøft
Meet Kate Lützhøft, the Associate Manager at our Insulin factory, our ALP production and our warehouse.
Read more
Looking back and looking ahead: An interview with Rasmus Hother le Fevre
We sat down with Rasmus Hother le Fevre, Managing Director and Corporate Vice President of Novo Nordisk Pharmatech, to talk about the impact of COVID-19, and look ahead at the strategic vision for 2021 and beyond.
Read more
Pharmaceutical grade Quats in accordance with cGMP Guide ICH Q7 for APIs
We manufacture our pharmaceutical grade Quats in accordance with the cGMP Guide ICH Q7 for Active Pharmaceutical Ingredients (APIs), the highest available quality standard in the industry.
Read more
Meet Kristoffer Laursen
Meet our Research & Development Director Kristoffer Laursen. Kristoffer is head of our R&D department which consists of more than 30 people.
Read more
A good start is half the work
New colleagues always start with a two-day onboarding program. This is to ensure a good beginning – regardless the type of employment.
Read more
Meet Gernot Stadlmann
Gernot is head of our Sales and Marketing department consisting of our experienced Customer Support team, Sales Managers, Product Managers, Product Development and Marketing.
Read more
Striving for zero environmental impact
Environmental challenges have never been more critical or more urgent than they are today.
Read more
How does Recombinant Insulin differ from animal-derived insulin
Recombinant Insulin, also known as Insulin Human AF, consists of human insulin crystals – a biosynthetic product produced by recombinant microbial expression in yeast.
Read more
Novo Nordisk Pharmatech will contribute to the scientific agenda at BPI Digital Week
Novo Nordisk Pharmatech will contribute to the scientific agenda at BioProcess International Digital Week (Dec 7-10) with a presentation on upgrading cell stem cultures.
Read more
Take a look inside our cGMP Quats manufacturing facilities
Being cGMP certified, we ensure that every aspect of our manufacturing process is in control – from our own suppliers to the finished product. We source the best raw ingredients, which are sampled to check they are in specification.
Read more
Get peace of mind for your full product life cycle
Novo Nordisk Pharmatech helps customers reduce their risk, with a high level of consistency in both the quality and supply of our Quats and Human Insulin AF.
Read more
A strong partnership with Thermo Fisher Scientific
“Novo Nordisk Pharmatech is a top performer across our supplier measurements. But it’s different from just supply and order – this is a partnership,” Vince Saint explains.
Read more
Reduce the risk for your raw materials with pharma-grade Quats
If you want to reduce the risk for your raw materials, pharma-grade Quats is the way to go – both for APIs and expients. We are specialists and a dedicated manufacturer of Quats in an unequalled, full cGMP grade.
Read more
Why use recombinant Insulin in DoE for your media development?
On October 6-7 we will make our debut at Cell Series UK virtual conferences. We are contributing to the Cell Culture and Bioprocessing conference track on Cell Media.
Read more
Join us at CPhI Festival of Pharma
We will maintain our longstanding relationship with CPhI as a Platinum Sponsor of the online event, sponsoring the keynote: “How will lessons learnt from COVID-19 shape new EU pharmaceutical policy”.
Read more
How can you mitigate your raw material risk, especially during a pandemic?
The COVID-19 crisis has reinforced the importance of having a strong supply chain and a risk-management and business-continuity plan. Which risks have you identified?
Read more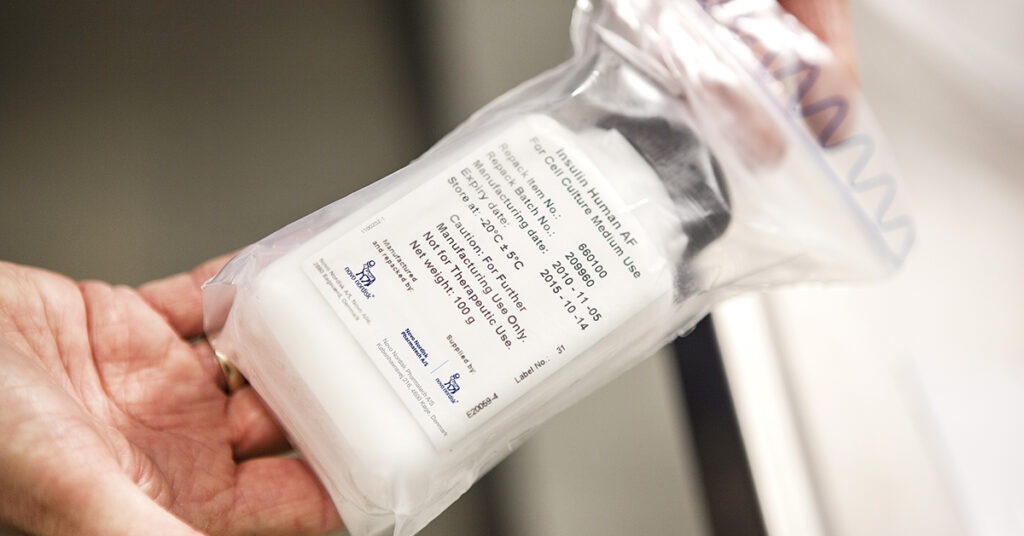
Avoid the risk of impurities with our Insulin Human AF
With Insulin Human AF, you avoid the risk of impurities such as mycoplasmas, which can pass line to line from the upstream bioreactor and contaminate your whole line.
Read more
It’s our responsibility to nurture the next generation of talent.
We believe it’s our responsibility to nurture the industry’s next generation of talent. But, equally, we also gain so much from our interns’ broad range of educational and cultural backgrounds.
Read more
Ensuring regulatory compliance with Brenntag Specialities
“Novo Nordisk Pharmatech’s Quats meet the European, US, British and Japanese pharmacopoeias, which is a great asset for our marketing.”
Read more
We are certified to ISO 45001
We are now certified to ISO 45001, the newest ISO standard within OH&S. ISO 45001 has become one of the most eagerly awaited standards in the world and is set to drastically improve levels of workplace safety.
Read more
Key component in serum free growth media for mammalian cells
Our recombinant insulin is a key component in serum free growth media for mammalian cells. It is used in many cell lines for the manufacturing of monoclonal antibodies, virus vaccines and gene therapy products.
Read more
Our employees can commute in a green way
As a natural consequence of our Circular for Zero environmental strategy we have established 10 parking spaces with charging stations for electric vehicles. This makes it even more attractive to commute in a green way.
Read more
Securing your end-to-end supply chain
Supply chain safety, quality and reliability are key to all our Quats and Insulin Human customers. Here is how we can help you secure your supply chain, from sourcing raw materials to delivering your products as promised – even in a global crisis.
Read more
Long seniority and high professionalism
Long seniority and high professionalism are both important cornerstones in our business. Almost half of our colleagues have a seniority above 10 years in the Novo Nordisk Group.
Read more
BioProcess International Europe 2020
Novo Nordisk Pharmatech is an active part of Europe´s leading Bioprocessing. You will here get much more information about our Insulin Human AF and how it can help you in your upstream processes.
Read more
Facing a pandemic as COVID-19
Our customers depend on us to deliver their needed products – even when we are facing a pandemic as COVID-19. While our customers depend on us, we depend on our employees.
Read more
A wide range of capabilities are available at Novo Nordisk Pharmatech A/S
A wide range of capabilities are available at Novo Nordisk Pharmatech A/S within the various departments; Sales and Marketing, R&D, Manufacturing, Quality, Business Support and HR & Communication.
Read more
Increase viable CHO cell density with recombinant Insulin Human AF
You can increase viable CHO cell density by supplementing with recombinant Insulin Human AF. CHO cells are one of the most widely used platforms to produce biopharmaceuticals.
Read more
How do we ensure the quality of materials and products?
What are the benefits of using cGMP Quats as excipients? And how does Novo Nordisk Pharmatech ensure to stay on top of the pace of technology change?
Read more
Novo Nordisk Pharmatech A/S has been a CO2 neutral company since 2018
We have zero CO2 emissions from energy consumption. In 2019 we also committed to the Novo Nordisk Circular for Zero environmental strategy. This means we will strive to have zero environmental impact by 2030.
Read more
We will develop our employees
We will develop our employees. This is one of our goals at Novo Nordisk Pharmatech. Therefore, from the first day we encourage our employees to think about their continuing education.
Read more
Extensive experience supplying Quats for the biopharmaceutical and pharmaceutical industries
Did you know that we at Novo Nordisk Pharmatech have 70 years of extensive experience supplying Quats (quaternary ammonium compounds) for the biopharmaceutical and pharmaceutical industries? Do you also know the story behind our company name?
Read more
Reliable supply of the highest quality ingredients for biopharmaceutical and pharmaceutical industries
Recombinant Insulin for cell growth media and pharmaceutical grade Quaternary Ammonium Compounds. Both manufactured to cGMP standards. Our proven record of precision delivery and product quality allows you to keep development on track and production flowing.
Read more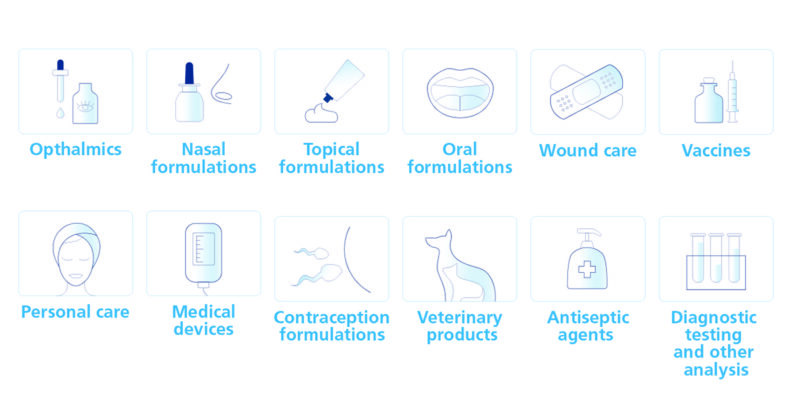
Our full cGMP processes make us the leading supplier for a wide range of applications
Proven under ICH Q1 conditions, our Quats are very stable. They are powerful antimicrobial surfactants, soluble both in aqueous and oily phases and basically active at all pH ranges. They can be used for a large number of applications requiring high purity and quality.
Read more
Almost four weeks has passed since the first COVID-19 announcement
Plans were made in regard to prioritizing within the frame set by the authorities and it was quickly evident that we are change ready and flexible in solving that task. This way we can still ensure that our customers will receive the same high-quality products as always.
Read more
Grow your cells with recombinant Insulin
We source our recombinant insulin directly from our mother company Novo Nordisk, the world’ s largest insulin producer. Thanks to our solid supply chain, you can keep development on track, production flowing, and products supplied to hospitals and patients.
Read more
Manage risk in downstream vaccine production
CTAB, called Cetrimonium Bromide or Cetyl Trimethyl Ammonium Bromide, is a powerful cationic surfactant for use in vaccine downstream purification steps.
Read more
COVID-19: Looking for an effective antimicrobial agent?
BKC is an ideal antimicrobial agent with proven efficacy. It is broadly used in topical and wound care products such as hand sanitizers, antiseptic liquids, foams, gels and cleansing wipes.
Read more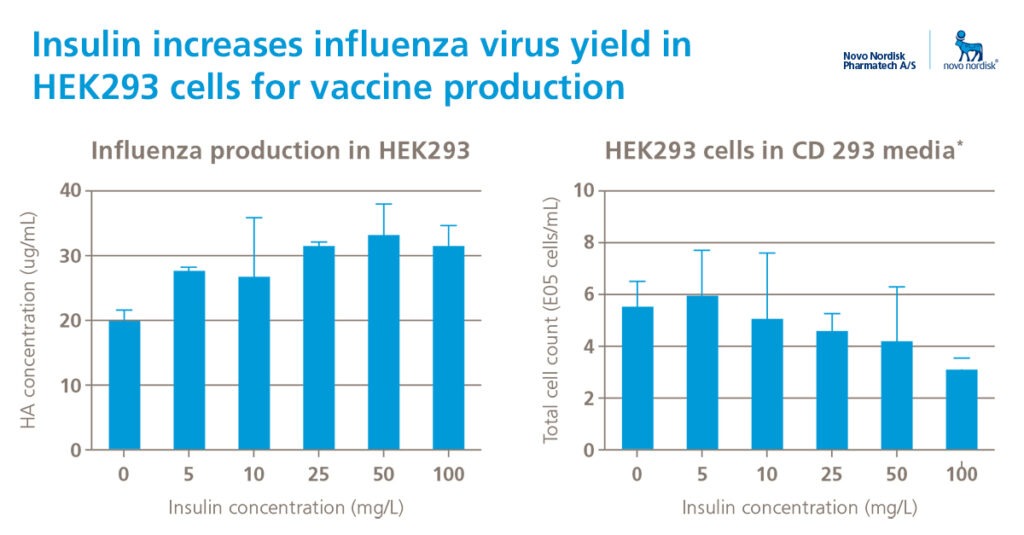
Increase VCD and specific viral yield
The vaccine industry is challenged to produce large quantities of vaccines in a rapid and cost-effective way. Major changes to current bioprocesses are both difficult and very expensive to implement.
Read more
Coronavirus´ impact on current product portfolio
Novo Nordisk Pharmatech A/S is continuously reviewing the likely impact upon its supply chain if product availability is affected by the consequences of the coronavirus.
Read more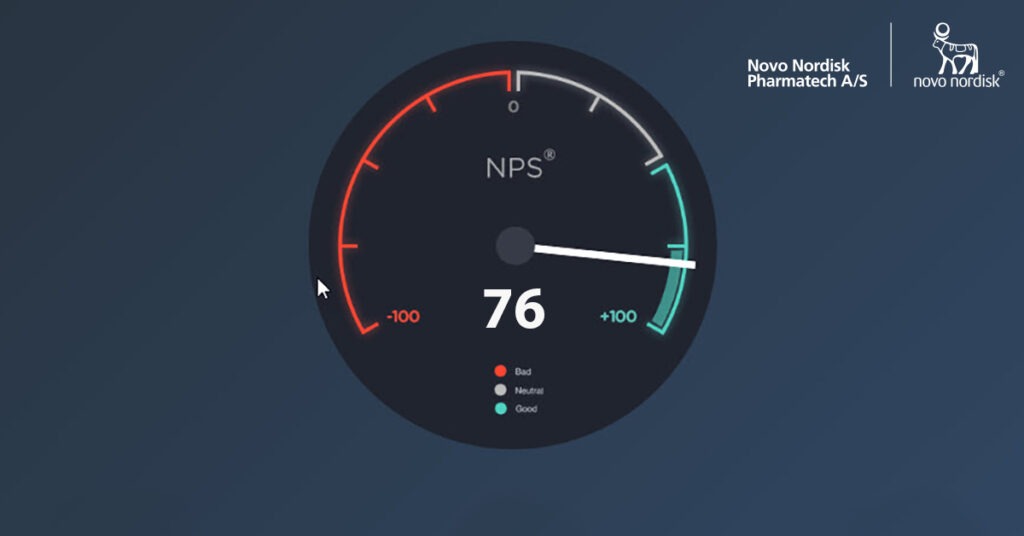
Our NPS score for 2019 ended at impressive 76
Since 2014 we have significantly improved our NPS score*, and in 2019 we had an impressive average score of 76. NPS scores range from -100 to 100.
Read more
Welcome our new Director of Manufacturing John Halfdan Boiesen
Our Director of Manufacturing will be heading up the manufacturing facility ensuring that we deliver high quality products in regulatory compliance. Part of the Manufacturing responsibility is to ensure that we have all necessary permissions to operate and operate with minimal environmental impact.
Read more
Chantale Julien has re-joined Novo Nordisk Pharmatech
We are pleased to announce that Chantale Julien has re-joined Novo Nordisk Pharmatech on January 1st, 2020. Chantale will be acting as Global Product Manager for the Quats and Insulin Human AF products.
Read more
New branch office in Singapore
The grand opening of Novo Nordisk Pharmatech A/S new branch office in Singapore on October 15th 2019 was celebrated together with customers, employees and other stakeholders.
Read more