Solutions
Recombinant Insulin
High-quality, animal-free, and reliable Recombinant Insulin produced by the world’s largest insulin manufacturer, supported by a dedicated team of insulin experts, and securely supplied around the globe.
What is Recombinant Insulin?
Recombinant Insulin (also known as Insulin Human AF) is a recombinant protein consisting of human insulin crystals, a biosynthetic product created using recombinant microbial expression in yeast. As insulin binds to its receptor, it initiates intracellular signalling pathways that regulate cell growth, differentiation, survival and glucose uptake. This promotes cell culture growth and enhances protein yields by modulating transcription, DNA synthesis and replication, and stimulating protein translocation.
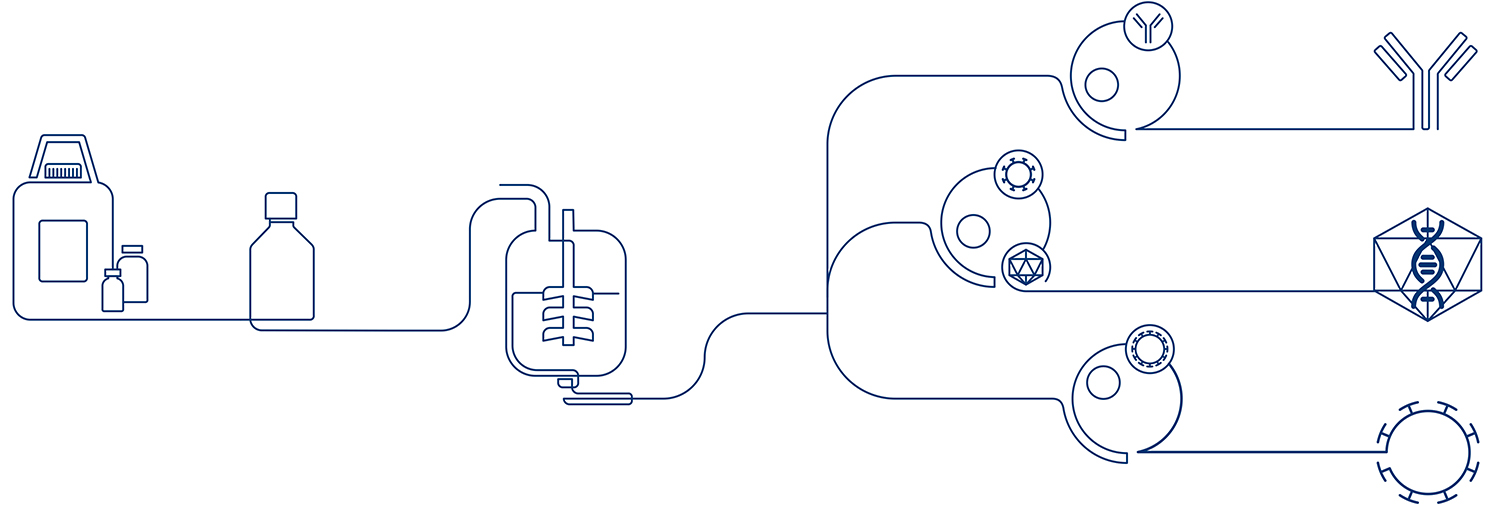
Recombinant Insulin is used to produce monoclonal antibodies, virus vaccines, gene therapy drugs, and other innovative biologic products approved for use by regulatory bodies worldwide.
Advantages of choosing Recombinant Insulin
High purity, recombinant, animal-free product
Batch-to-batch consistency
Reliable and experienced partner, with +100 years experience in insulin
Secure improved cell growth and protein yield
Following global regulatory compliance
Recombinant Insulin applications
Our Recombinant Insulin is a key component in serum free growth media for mammalian cells. It is used as supplement in the cell culture media for several cell lines, such as CHO, HEK293 or Sf9, for the manufacturing of monoclonal antibodies, virus vaccines, or gene therapy products.
Product list
Get a full overview and find the right solution that makes your process or product better.
Contact our Customer Support to place your order here.
Enabling better medicines
We provide excellence at every step of the supply chain, beginning with the quality of our insulin, which is directly sourced from Novo Nordisk A/S, the largest producer of insulin in the world. More than 100 years of experience ensure that we have the most stable manufacturing processes and the most sophisticated subject knowledge available – making us the market-leading supplier of insulin for cell culture processes.
A guide to using Recombinant Insulin to enhance cell culture growth
Produced naturally by the body, growth factors function to regulate cell division and cell survival in vivo. They can be biosynthetically produced and have a strong history of use in the pharmaceutical industry, where they help manufacturers to optimise yields of biologic drug products. A prime example is insulin – a key component in serum-free growth media for mammalian cells.
Insulin is part of a family of growth factors, including Insulin-Like Growth Factors IGF 1 and IGF 2. Novo Nordisk Pharmatech’s Recombinant Insulin is a recombinant insulin product used by biopharmaceutical companies worldwide in the production of monoclonal antibodies, virus vaccines, and gene therapy drugs, plus a range of other innovative biologic products.
As insulin binds to a receptor, it initiates different intracellular signalling pathways involved in regulating cell growth, cell differentiation, cell survival, and cellular glucose uptake. By modulating transcription, DNA synthesis, and replication, and stimulating protein translocation, insulin promotes cell culture growth and enhances yields.
Recombinant Insulin is best-used in serum-free (chemically defined) media. Let’s take a look at some examples.
What does the study say?
Important proteins in the development of oncology treatments, monoclonal antibodies (mAbs) are most often produced in CHO cells. With the application of Recombinant Insulin, data has shown up to a 66% yield increase in mAb production, alongside increases in cell viability and density.
What you should know
If using commercially available media, it is important to know the full contents of the media, including whether insulin is already contained. Using this information, manufacturers will most likely need to optimise the media to their own cell line and the desired end-product. This can involve optimising the insulin level by doing trials at different concentrations. Based on our own published data, Novo Nordisk Pharmatech is happy to guide customers on which insulin concentrations will produce the highest yields for their desired cell line.
If you decide Recombinant Insulin is the right growth factor for your cell culture, the next step is to ensure you source it from a reliable, fully qualified supplier. It is also important to use ingredients with a high batch-to-batch consistency, helping you keep better control of your processes. Our Recombinant Insulin is manufactured by our parent company, Novo Nordisk, and supplied to patients around the world for therapeutic use. Manufactured in cGMP facilities and following an ISO 9001 quality system, the product is produced in accordance with the highest requirements on the market.
Recombinant Insulin comes in a dry crystalline form, which can be dissolved in water. It has a long shelf life of five years but must be stored and handled carefully across this time. To ensure Insulin Human AF stays within product specifications, it should be stored at freezing temperature, below -20°C. However, the product can tolerate being held in refrigerator or room temperature for limited periods of time.
If you need instructions for dilution, you can find our information sheet here.
Additional resources
Explore more about Recombinant Insulin
Insulin insights
Explore our collection of materials and case studies about Insulin Human AF and see how it improves cell culture and boosts protein production.

Insulin 10 reasons why
Reduce the risks of contamination with our Benzalkonium Chloride that’s the best and safest antimicrobial for the pharmaceutical, medical device and personal care industries.
Insulin applications
Explore the many applications and learn how it can help support your formulations.



