Webinar details
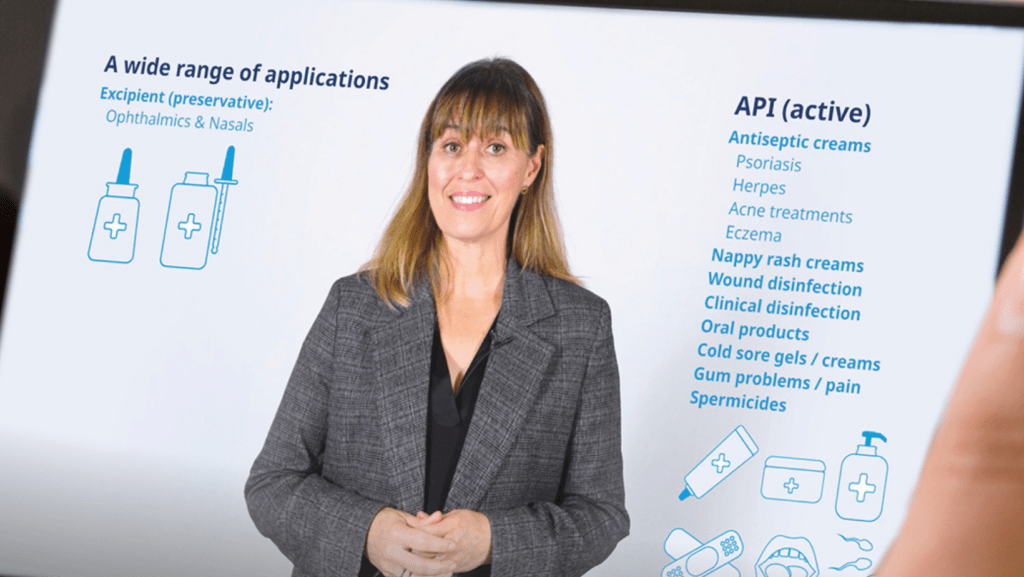
As pharmaceutical formulations become more complex, manufacturers are under increasing pressure to deliver safe, stable products without introducing unfamiliar ingredients. Proven excipients like Benzalkonium Chloride (BKC) offer a dependable solution—providing strong antimicrobial control while supporting formulation stability and multi-dose shelf life.
In this webinar, we will share data-driven insights on BKC’s performance, including comparative analyses with other antimicrobials and guidance on establishing effective, patient-safe concentrations. You will learn how BKC’s long history in ophthalmic, nasal, and topical products translates into broad-spectrum efficacy, minimal irritation, and reliable formulation support.
Designed for professionals in formulation, development, quality, regulatory affairs, and procurement, this session will explore why BKC remains a versatile, trusted option for modern pharmaceutical applications and how it can enhance both product safety and stability.
Watch the webinar
Speaker

Chantale Julien, Global Product Manager
With over 20 years of experience in the pharmaceutical industry, Chantale is currently a Global Product Manager at Novo Nordisk Pharmatech A/S, where she manages several product portfolios.
Her role encompasses providing technical and scientific support, strategic market positioning and adherence to GXP requirements.
Throughout her career, she has held positions at the Canadian Food Inspection Agency, Novonesis (previously Chr. Hansen A/S) and Cook Medical, in various management roles that emphasize compliance, life-cycle product management and API distribution.
Mrs. Julien earned her Master’s degree in Animal Sciences from Université Laval in Québec, Canada.