Solutions
TrypsiNNex®
Animal-free, recombinant porcine Trypsin
What is TrypsiNNex®?
Advantages of choosing TrypsiNNex®
Animal-free
Eliminate the risk of viral contamination from raw material
High purity
Achieve high process consistency owing to consistently high β-trypsin content
High quality
Easy qualification or transition from your existing trypsin product, supported by extensive documentation and support from a preferred pharma grade supplier
The animal-free, recombinant TrypsiNNex® sequence is identical to porcine Trypsin and can be used as a direct replacement when converting to animal-free manufacturing. TrypsiNNex® is expressed in E. coli as an inactive product. The trypsin remains inactivated during both the upstream and downstream manufacturing process, minimizing the level of auto degradation before final stabilization and packaging, thereby preserving a high content of β-trypsin.
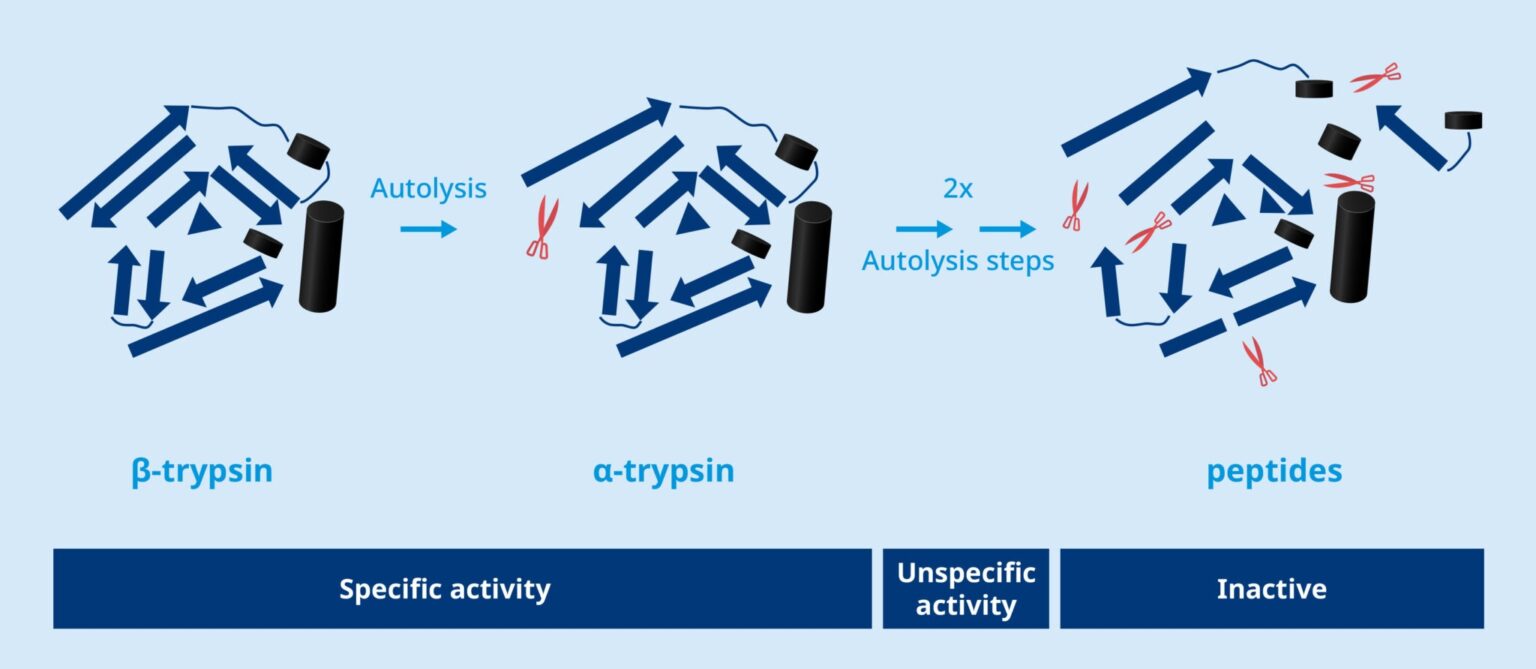
Trypsin has intrinsic autolytic activity and is degraded in steps, where β-trypsin is converted into α-trypsin through proteolytic cleavage (red scissors).
This process continues until the trypsin is inactivated. Intermediate forms such as Ψ-trypsin display unspecific activity, which is undesirable. Thus, a high β-trypsin content is a quality indicator that ensures consistent activity and high specificity.
TrypsiNNex® has a measured β-trypsin of typically well above the minimum specifications at 70% ensuring a high activity and consistent proteolytic pattern, which is critical when processing precursor proteins, such as pro-insulin.
TrypsiNNex® applications
TrypsiNNex® is used for three main applications:
Product list
Get a full overview and find the right solution that makes your process or product better.
Contact our Customer Support to place your order here.
Product information
TrypsiNNex® is GMP manufactured in accordance with IPEC’s guideline “The Joint Good Manufacturing Practices Guide for Pharmaceutical excipients” (2017) and applicable chapters in pharmacopoeias and ICH guidelines.
Enzymatic activity*: >333.000 USP units/ml
Product purity : ≥ 90 % (α- and β-trypsin)
β-trypsin content: ≥ 70%
Protein content: 55-85 mg/mL
Formulation: 10 mM HCl, 20 mM CaCl2
Bioburden (Microbial Count): ≤ 10 CFU/mL
Stability: 36 months at –20ºC ±5ºC
*Alternative units
1 Chromozym TRY Unit: 21 USP Units
(Source: www.omicsonline.org)
- Certificate of analysis
- ISO 9001, 14001, 45001 certificates for Novo Nordisk Pharmatech’s management systems
- TrypsiNNex® user guide
- Quality statements: Origin/TSE, impurities, stability, primary packaging, manufacturing, etc.
- Regulatory Support File
- Supplier questionnaire
- SDS
- EcoVadis Sustainability report
- Novo Nordisk Pharmatech’s quality system meets DS/EN ISO 9001 and Novo Nordisk Pharmatech’s environmental systems meet DS/EN ISO 14001 and 45001.
- TrypsiNNex® is manufactured in accordance with GMP as defined by Novo Nordisk Pharmatech based on applicable chapters in DS/EN ISO 9001 and IPEC GMP guidelines for excipients (current version).
- TrypsiNNex® is produced by recombinant microbial expression in E.coli. No ingredients of animal origin are used in the cell banking and manufacturing process.
- TrypsiNNex® is antibiotic-free, both in production facilities and on a cell bank level.
- TrypsiNNex® is animal-free, meaning it is TSE compliant
Other practical information
TrypsiNNex® is shipped on dry ice.
TrypsiNNex® should be stored at ‑20°C ±5ºC immediately upon arrival.
Please contact us for information on storage and handling after dilution.
If trypsin inhibitors are needed, we recommend using regular animal-free trypsin inhibitors, e.g. a Soybean Trypsin inhibitor.
In Biopharmaceutical production, detection of raw material is necessary:
- Incoming testing of TrypsiNNex® (e.g. identity, activity)
- Detection of residual raw material in the finished product (LOQ for HPLC-based purity measurement)
For support on incoming quality testing, please contact us.
Additional resources
More about TrypsiNNex®
TrypsiNNex® applications
TrypsiNNex® specifically addresses the need for a consistent, high-purity and high-quality enzyme in biopharmaceutical manufacturing.