Solutions
Benzalkonium Chloride
10 reasons why
High GMP quality
Our pharmaceutical BKC is manufactured in accordance with cGMP Guide ICH Q7 for Active Pharmaceutical Ingredients, the highest available standard.
We analyze according to relevant multicompendial pharmacopoeia: Ph.Eur., USP/NF, JP, and ChP.
Our quality performance is continuously monitored by both customers and authorities, and we strive to continuously improve based on audit feed-back, industry trends and customer centricity.
High purity
A fully controlled synthesis process based on 70 years’ experience in manufacturing Benzalkonium Chloride and 30 years’ experience with GMP, results in high levels of purity, making our Benzalkonium Chloride products particularly suited for pharmaceutical applications.
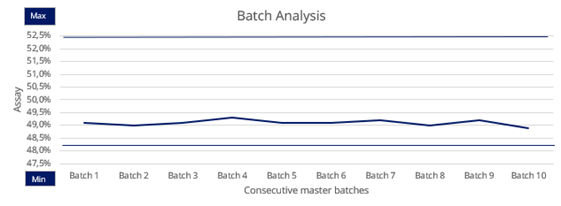
The high purity level is highly consistent from batch to batch, thanks to our risk-based approach and fully validated processes.
Consistency
Our stable and well-established manufacturing, analytical and quality processes ensure continuous product consistency and availability. Therefore we deliver as promised, on-spec and on time.
The cornerstone of a quality organisation is an effective quality management system. We are certified ISO 9001 and GMP according to ICH Q7 guideline for APIs, and we comply with all relevant quality ICH guidelines such as Q1, Q2, Q3A, Q9, Q10, & Q11.
The same quality, batch after batch
Reliability
Manufacturing know-how and GMP experience are some of the founding blocks assuring our reliability as a manufacturer and supplier.
We are a pharma ingredient manufacturer dedicated to serve the pharma manufacturing industry.
Every day, we value our partners and customers and strive to exceed industry expectations.
Our customer’s feed-back speaks for itself. When asked “Why did you choose our products”, customers respond:
- “You are the incumbent supplier”
- “The product (red.) is going into more than one of our brands and we never had delivery issue, always on time”
- “Product quality and availability”
- “We have chosen your products for their quality and for the regulatory package which is precise and regularly updated”
- “Quality products with compliant documentation”
Global regulatory compliance
Novo Nordisk Pharmatech is regularly audited by major and minor pharmaceutical companies and inspected by the Danish Medicine Agency.
Our comprehensive documentation and services include:
- ISO 9001 Certificate
- GMP Certificate from the Danish Medicine Agency
- Certificate of Analysis (current version of pharmacopoeias)
- Customer audits
- Declarations, statements (stability, residual solvents, TSE/BSE, nitrosamines, allergens, elemental impurities, etc.)
- Change Notification
- Supplier questionnaire
- Process flowchart
- Packaging details
- Quality assurance agreements on Novo Nordisk Pharmatech template
Wide range of applications
A proven record of efficacy against a broad spectrum of microorganisms makes our Benzalkonium Chloride (BKC) very effective for a wide range of therapies, administration routes and product forms.
BKC is well established for use in:
- Ophthalmic and nasal formulations
- Topical products
- Oral/dental and respiratory products
- Medical devices for wound care
- Veterinary products
and other applications that require a high purity and quality antimicrobial excipient or API.
Embracing a circular mindset
Our environmental strategy, Circular for Zero, sets out targets and milestones for the next 10 years of this journey. Our ambition is bold and simple: to have zero environmental impact.
We will collaborate proactively with suppliers to embed a circular mindset for reduced environmental impact across our value chain and switch towards circular sourcing and procurement.
We will eliminate the environmental footprint from our operations and drive a circular transition across the company to achieve our zero environmental impact ambition.
We will upgrade existing and design new products based on circular principles and solve the end-of-life product waste challenge to close the resource loop.
Sustainability and triple bottom line
Novo Nordisk is a member of the UN Global Compact and World Business Council for Sustainable Development (WBSCD). As a fully owned subsidiary, we follow the general policies of Novo Nordisk for sustainability, business ethics and code of conduct.
We believe that a healthy economy, environment and society are fundamental to long-term business success. This is why we manage our business in accordance with the Triple Bottom Line (TBL) business principle, conducting activities in a financially, environmentally and socially responsible way.
Our track record in the pharma industry
More than 75 years manufacturing Benzalkonium Chloride and 30+ years’ experience with pharma cGMP guarantees the best solution for your application.
Our own highly qualified personal work continuously on product development and improving documentation to suit your needs.
This expertise – together with product and documentation availability and global compliance – has made Novo Nordisk Pharmatech a preferred supplier with pharmaceutical companies worldwide.

Want to learn more?
For more information about our Quats products, please contact us or visit our Quats product page.
Explore more about Benzalkonium Chloride

Benzalkonium Chloride (BKC)
Reduce the risks of contamination with our Benzalkonium Chloride that’s the best for ophthalmic, nasal, topical and oral applications.
BKC insights
Discover webinars, videos and whitepapers showcasing the broad antimicrobial effects of BKC against bacteria, yeast, mold, and enveloped viruses.
BKC applications
Quaternary Ammonium Compounds, also known as BKC, have a long history of use in the pharmaceutical industry, where their broad antimicrobial effect against bacteria, yeast,
