Adherent cell culture systems are vital in biomanufacturing, serving as a foundation for development and production of various vaccines and therapeutics. As a key cell dissociation reagent that enables culture expansion, trypsin can impact the efficiency and scalability of a production process.
Our recombinant enzyme TrypsiNNex® was designed as a high-purity, animal component-free cGMP trypsin that is easily integrated into existing and new protocols for cell culture passaging. TrypsiNNex® enable efficient and robust dissociation of various continuous adherent cell lines utilized in the pharmaceutical production including vaccines, and for mesenchymal stem cell-based therapies providing homogenous and viable cells
Learn more from our application study on efficient cell dissociation using TrypsiNNex®:
Performance assessment and strategy for using the new recombinant trypsin TrypsiNNex® in passaging HEK293, MDCK, MRC5, BM-MSC, UC-MSC, and AD-MSC cell lines.
Consistency is an essential aspect of biopharmaceutical manufacturing that is impacted by the quality and performance of the raw materials used. Also, the uniformity of enzymes, like trypsin, that are used to manage adherent cell cultures, contribute to achieving a consistent product quality.
The study assesses the performance and efficiency of TrypsiNNex® in cell dissociation and profiles its activity and compositional stability over time. Stored at 4°C, a 70 U/mL working solutions of TrypsiNNex® demonstrate the stable composition and activity with a high proportion of β-trypsin for at least 30 days. In fact, β-trypsin exceeded α-trypsin in the solution for up to 6 months with some decline in activity due to the gradual autolysis. Additionally, a TrypsiNNex® stock solution tolerated at least 5 freeze-thaw cycles without changes in composition or performance.
As a cell dissociation reagent, TrypsiNNex® proved to be equivalent to commonly used commercial trypsin products. These experiments provide guidelines for the use of TrypsiNNex® with different cell lines, outlining enzyme concentrations and incubation times that favour efficient passaging and high numbers of viable cells.
Trypsin quality is essential in biopharmaceutical production
Robust biopharmaceutical production is ensured by robust raw materials. In the case of manufacturing processes that rely on adherent living cells as producers, trypsin is a particularly influential input. Used optimally, trypsin efficiently detaches cells from their growth support during passaging, allowing continued population expansion. Used in excess, however, trypsin cleaves surface proteins and can curtail cell viability and replication (1).
Whether used in virus propagation for vaccines (2), therapeutic protein expression, or vector manufacturing for cell and gene therapies (3), the reliable performance of trypsin is pivotal to facilitating rapid and reproducible cell population growth whether used in cell expansion or cell dissociation or protein modification. Trypsin used in the manufacturing of medicinal products must exhibit predictable activity throughout a reasonable shelf life while being free of any agents that can potentially contaminate or infect the producer cells.
This study examines the performance of TrypsiNNex®, a new recombinant trypsin, in the adherent cell dissociation. We first describe the key features underlying its enzymatic activity and safety profile.
Then, we test the stability of its composition and activity over time and through freeze-thaw cycles. Finally, we compare the efficiency of TrypsiNNex® to other commercially available trypsin products in passaging different cell lines commonly used in biopharmaceutical manufacturing.
TrypsiNNex®: Delivering reliability and consistency through purity
TrypsiNNex® is designed to perform consistently and, thus, close a significant gap in the narrow performance tolerances for biomanufacturing raw materials. That consistency is achieved by enforcing two aspects of purity. The first aspect is the animal component-free production of TrypsiNNex®. Viruses and other adventitious agents that have caused costly contamination events in processes using animal- derived trypsin are averted through recombinant production and stringent quality control. This means that TrypsiNNex® can be integrated into new and existing manufacturing processes to eliminate safety risks (see highlight box below “The risk of viruses in raw materials”).
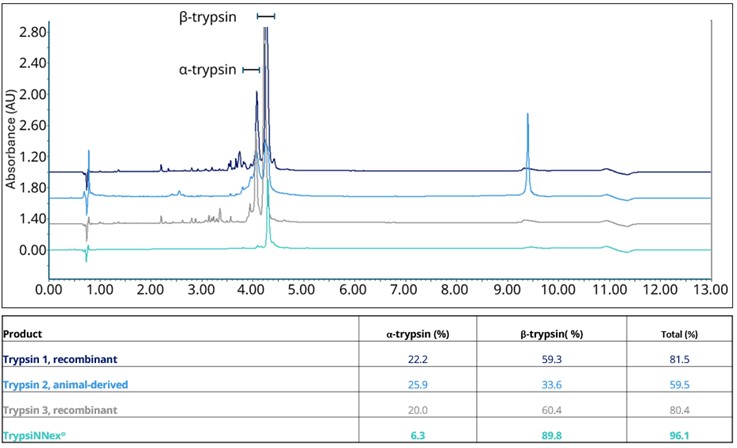
The second aspect is the high purity and consistent proteolytic activity of each TrypsiNNex® lot. Variation in enzymatic activity is regularly observed within and among lots of commercial trypsin products. A common culprit of those deviations is autolysis. Trypsin is an unstable molecule and self-degrades, producing decomposition fragments with reduced activity and altered specificity (5). As a result, trypsin products are a mixture of fully active enzyme (β-trypsin), subpar enzymes (e.g., α-trypsin), and other components. This autodegradation can downgrade the overall performance of a mixture and lead to differences among enzyme lots depending on reaction conditions (6).
To control the composition of TrypsiNNex® lots, autolysis during production is restricted. TrypsiNNex® is expressed in Escherichia coli cultures as an inactive protein subsequently activated during purification. With stabilization shortly thereafter, premature degradation is averted, and a substantial proportion of intact trypsin (β-trypsin) is safeguarded. In fact, the overall purity of TrypsiNNex® measured via reversed-phase high-performance liquid chromatography (RP-HPLC) exceeds 90%. The amount of β-trypsin in TrypsiNNex® is at a minimum 20 percentage points higher than in other tested commercial trypsin products relative to total HPLC absorbance at 215 nm (Figure 1).
Whether used in virus propagation for vaccines (2), therapeutic protein expression, or vector manufacturing for cell and gene therapies (3), the reliable performance of trypsin is pivotal to facilitating rapid and reproducible cell population growth whether used in cell expansion or cell dissociation or protein modification. Trypsin used in the manufacturing of medicinal products must exhibit predictable activity throughout a reasonable shelf life while being free of any agents that can potentially contaminate or infect the producer cells.
This study examines the performance of TrypsiNNex®, a new recombinant trypsin, in the adherent cell dissociation. We first describe the key features underlying its enzymatic activity and safety profile.
Then, we test the stability of its composition and activity over time and through freeze-thaw cycles. Finally, we compare the efficiency of TrypsiNNex® to other commercially available trypsin products in passaging different cell lines commonly used in biopharmaceutical manufacturing.
The risk of viruses in raw materials
Trypsin is often sourced from porcine or bovine pancreatic tissue. Though typically less expensive, animal- derived trypsin carries the risk of harboring viruses and other adventitious agents that can adversely affect cell lines or the safety of a final product. The consequences of an infected cell line can be millions of dollars in production downtime, extensive corrective action, limited supply of much-needed medicines, and the danger of infecting patients. Furthermore, an industry-wide study of viral contamination risk in manufacturing processes highlighted that testing alone is insufficient to guarantee a product free of introduced viruses (4).
Robust performance over time and repeated freeze-thaw cycles
TrypsiNNex® should be diluted before use. While the shelf life of the stock solution is 3 years when stored at –20°C, the stability of a working solution is an important consideration in process development.
Changes in the composition and activity of TrypsiNNex® working solutions stored at 4°C (Figure 2) and 21°C (data not shown) were tested over time. Two working solutions were created by diluting TrypsiNNex® in phosphate buffer saline (PBS) with 1 mM EDTA to 70 U/mL and 700 U/mL. The working solutions were stored at the test temperatures and sampled at different time points for up to 6 months. Total trypsin and the proportion of β-trypsin versus α-trypsin were profiled via reversed-phase high-performance liquid chromatography (RP-HPLC). The proteolytic activity of the TrypsiNNex® working solutions was measured as the change in absorbance per unit time (defined in USP <89>) resulting from the hydrolysis of the Chromozym TRY substrate (CustomBiotech, Germany).
Although the total trypsin measured remained relatively constant over time, TrypsiNNex® degraded in PBS with EDTA. The ratio of β-trypsin to α-trypsin declined as intact trypsin autolyzed into increasing amounts of α-trypsin. The rate of autolytic decomposition, however, was decelerated at the lower enzyme concentration and storage temperature. Stored at 4°C, the 70 U/mL working solution retained more intact β-trypsin than α-trypsin for more than 6 months. Importantly, the initial ratio was stable for approximately 30 days (Figure 2A) before showing the first signs of degradation. In the 700 U/mL working solution, degradation occurred from the beginning and the ratio flipped in favor of α-trypsin after roughly 30 days (Figure 2C). Temperature had a strong effect on compositional stability, with both working solutions exhibiting a predominance of subpar α-trypsin within a few days after storage at 21°C (data not shown).
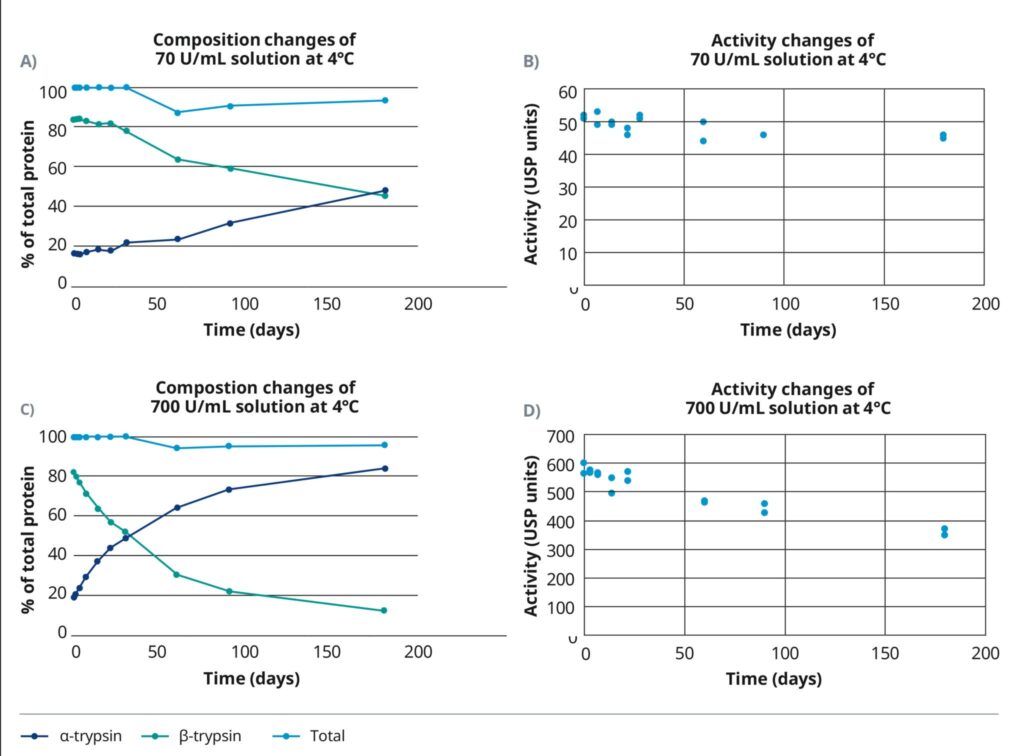
The observed shift from β-trypsin to α-trypsin was accompanied by an expected decay in proteolytic activity (Figure 2B, 2D). With 38% activity loss over 6 months, the decline was more pronounced in the 700 U/mL working solution. The less concentrated working solution exhibited only a 12% decline in the same timeframe (Figure 2B). Based on these results, we recommend storing working solutions of TrypsiNNex® at 4°C and using them within 14 days where composition remains steady and activity remains high. A 2-week window where a TrypsiNNex® working solution retains consistently high β-trypsin and proteolytic activity provides flexibility in manufacturing processes.
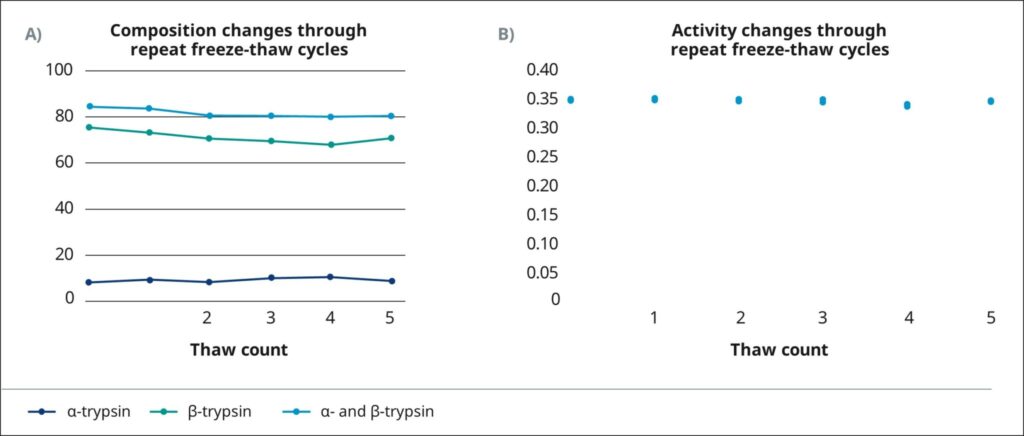
We also assessed the effect of repeated thawing on the quality of TrypsiNNex®. We thawed a stock solution in an ice bath, collected a sample for RP-HPLC analysis and activity assessment, and then refroze the stock solution at –20°C. We repeated this freeze-thaw cycle 5 times. Figure 3 summarizes the results of all thaw events. TrypsiNNex® had consistently ³80% total trypsin, with β-trypsin comprising >70% and α -trypsin about 10% of the stock solution. Proteolytic activity was also rigorously consistent across all freeze-thaw cycles. TrypsiNNex® stock solution can be safely thawed and refrozen repeatedly to create working solutions as needed.
Storage
We recommend storing TrypsiNNex® working solutions at 4°C. With a concentration of 70 USP/mL, the proportion of β-trypsin exceeds α-trypsin and proteolytic activity remains stable for 6 months. At 700 USP/mL, that stability is retained for 1 month. TrypsiNNex® stock solutions tolerate at least 5 freeze-thaw cycles without compositional changes or activity loss.
TrypsiNNex® effectively dissociates different cell lines
The efficacy of TrypsiNNex® as a cell dissociation reagent was tested on 6 cell lines: human embryonic kidney cells (HEK293), Madin-Darby canine kidney cells (MDCK), human fetal lung fibroblast cells (Medicinal Research Council strain 5, MRC5), bone marrow-derived mesenchymal cells (BM-MSC), umbilical cord-derived mesenchymal cells (UC-MSC), and adipose-derived mesenchymal cells (AD-MSC). Cells were seeded into 12-well plates or PET-based macrocarriers for 2-dimensional or 3-dimensional culture, respectively. After reaching 90% confluence, the cells were rinsed with PBS and incubated with different trypsin products. Depending on cell line, different concentrations and incubation times were tested. Trypsinization was stopped with culture medium and dissociated cells were counted and assessed for viability using a NucleoCounter® NC-200™ (Chemometec, Denmark). All experiments were conducted by an external laboratory.
TrypsiNNex® performed at the same level as other commercial trypsin products as a cell dissociation reagent in detaching cells from culture surfaces while maintaining high cell viability. Table 1 summarizes the results of the experiments, highlighting the concentration and incubation time that maximized viable cell density and percent viable cells after trypsinization with each tested trypsin product.
A description of the experimental methods and more detailed results are provided in the supplementary material “Efficient and consistent dissociation of adherent cell cultures using TrypsiNNex®“ (click to download).
In summary, TrypsiNNex® at 200 U/mL efficiently dissociated HEK293 cells and achieved over 90% cell viability after cell dissociation for nearly all tested incubation times (see supplementary document).
As with the benchmark trypsin products, a shorter incubation tended to lower cell viability and increase variation in viable cell density. Extending incubation to 5 or 10 minutes allowed for maximum cell dissociation without sacrificing viability. TrypsiNNex® effectively detached HEK293 cells with a 5-minute incubation, performing on par with the other commercial trypsin products used for cell dissociation.
A 10 and 20-minute incubation period allowed efficient dissociation of MDCK cells by all tested trypsin products, including TrypsiNNex®. Cell viability was uniformly above 90%, and post-dissociation monitoring showed good cell recovery (data not shown). The 5-minute incubation period produced results that were often below the detection limit of the cell counter used. Therefore, measurements of cell viability were not accurate in those cases. Finally, TrypsiNNex® was also highly effective in as cell dissociation reagent in 2-dimensional cultures of MSCs and MCR5 cells, performing at the same level as other commercial trypsin products.
TrypsiNNex® concentrations of 30 and 300 U/mL were highly effective at detaching cells from PET-based macrocarriers for all 3-dimensional (3D) cell cultures tested – mesenchymal stem cells (BM-MSC, AD-MSC, UC-MSC) and MRC5 cells (see supplementary document). At these concentrations, TrypsiNNex® performed well within the range of commonly used commercial trypsin products.
In some instances, the addition of EDTA to TrypsiNNex® improved results, though changes were not substantial. However, post-harvest monitoring showed that high concentrations of EDTA decreased cell viability, especially in MRC5 cells (data not shown). We advise diluting TrypsiNNex® in PBS with a low concentration of EDTA when dissociating MRC5 cells.
Recommendations
We recommend the following incubation times and TrypsiNNex® concentrations to passage 2D cultures of cell lines tested in this study:
- 2 minutes in 30 U/mL TrypsiNNex® for BM-MSC and UC-MSC
- 2 minutes in 300 U/mL TrypsiNNex® for AD-MSC
- 4 minutes in 300 U/mL TrypsiNNex® for MCR5 cells
- 5 minutes in 200 U/mL TrypsiNNex® for HEK293 cells
- 10 minutes in 1000 U/mL TrypsiNNex® for MDCK cells The addition of low-concentration EDTA can improve
To passage 3D cell cultures, we recommend:
- 30 minutes in 300 U/mL TrypsiNNex® for BM-MSC, UC-MSC, and MCR5 cell lines
- 30 minutes in 30 U/mL TrypsiNNex® for AD-MSC
The addition of low-concentration EDTA can improve results.
Under the conditions listed above, the performance of TrypsiNNex® in dissociating cells is comparable to other commercial trypsin products.
References
- Huang, H-L. et al. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J. Biomed. Sci. 17: 36. doi: 10.1186/1423-0127-17-36
- Genzel, et al. 2014. Vaccine production: upstream processing with adherent or suspension cell lines. Methods Mol. Biol. 1104: 371–393. Doi: 10.1007/978-1-62703-733-4_23
- Tan, et al. HEK293 cell line as a platform to produce recombinant proteins and viral vectors. Front. Bioeng. Biotechnol. 9: 796991.doi: 10.3389/fbioe.2021.796991
- Barone, et al. 2020. Viral contamination in biologic manufacture and implications for emerging therapies. Nature Biotechnol. 38: 563–572. doi: 10.1038/s41587-020-0507-2
- Heissel, et al. 2019. Enhanced trypsin on a budget: stabilization, purification, and high-temperature application of inexpensive commercial trypsin for proteomics applications. PLoS ONE 14: e0218374. doi: 10.1371/journal.pone.0218374
- Rosa, et al. 2021. Evaluation of biological activities, structural and conformational properties
Download application note
Download application note
Request quotation
Request a sample