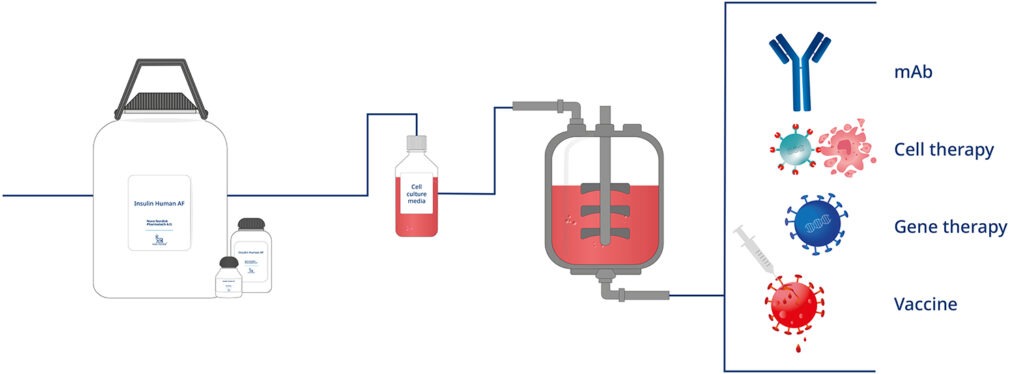
Our Recombinant Insulin, also known as Insulin Human AF, is a key component in serum-free growth media for mammalian cells
Recombinant Insulin





We guarantee
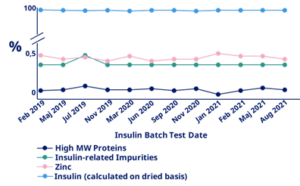
We live up to the highest quality standards, with a quality management system complying with DS/EN ISO 9001 for all activities. Our recombinant Insulin Human AF is manufactured in accordance with Novo Nordisk and Novo Nordisk Pharmatech quality systems, based on ISO 9001 and fulfils all relevant European and USA regulatory guidelines. It is produced under cGMP compliance and analysed according to the current European Pharmacopoeia (Ph. Eur.) and United States Pharmacopoeia (USP). Both Novo Nordisk and Novo Nordisk Pharmatech are regularly audited by major and minor pharmaceutical companies, as well as health authorities.
Our Recombinant Insulin is produced in yeast. This organism is neither stored nor grown in animal-containing media and does not contain genes or express antigens of livestock or poultry disease agents. Our recombinant insulin meets major cell culture media suppliers’ requirement for animal-free products. No animal origin component is used in the cell bank and manufacturing process. The trypsin used during insulin manufacture is replaced by a proprietary process aid of non-animal origin. Additionally, our insulin is antibiotic free. Insulin Human AF complies with ICH Q3C Note for Guidance on residual solvents.
With 100 years of experience with Insulin in 2023, Novo Nordisk ensures the most stable and well-established manufacturing processes and specialist know-how on the market. We are specialized in supplying ingredients to the biopharmaceutical and pharmaceutical industries and we deliver as promised, on-spec and on time. Our Recombinant Insulin is used by global large biopharmaceutical companies for the manufacture of their blockbuster drugs.
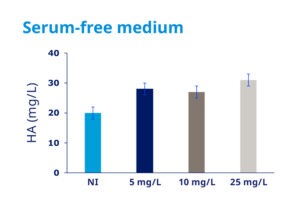
Recombinant Insulin is a key component in serum-free growth media for mammalian cells, as it stimulates the proliferation of cells. Recombinant Insulin is used as supplement in the culture media for many cell lines, such as CHO, HEK 293 or Sf9, for the manufacturing of monoclonal antibodies, virus vaccines, or gene therapy products, for example. Recombinant Insulin can also be used to produce other biological drug products approved by regulatory bodies worldwide, including FDA and EMA.
Discover the benefits of adding recombinant insulin to cell culture media:
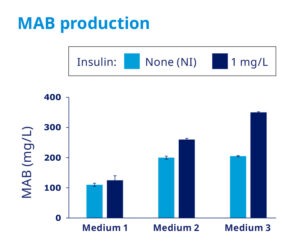
Recombinant Insulin increases mAbs yields in CHO cells
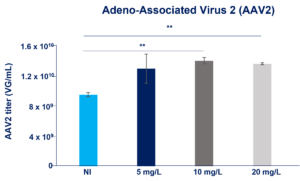
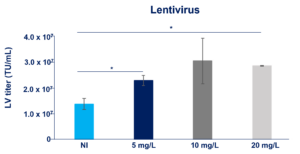
Recombinant Insulin enhances AAV and LV viral vectors production (link to the poster or insulin insights)
Recombinant Insulin increases Influenza Virus production in HEK293 cells
Recombinant Insulin improves HIV VLP production in insect Sf9 cells
We offer a comprehensive list of documentation and services:
- ISO 9001 certificate
- Certificate of Analysis (current versions of Ph.Eur. And USP/NF pharmacopoeias)
- Customer audits
- Declarations, statements (stability, residual solvents, TSE/BSE, GMO, allergens, etc.)
- Change Notification
- Pre-filled Supplier Questionnaire
- Process flowchart
- Packaging details
- Quality assurance agreements
- Certification as known consignor supplier
Every successful product needs a secure supply chain. Novo Nordisk Pharmatech A/S can help by ensuring dependable availability and a continuous supply of products. Our customers can rely on our robust risk mitigation strategy. Our entire manufacturing and quality control take place in Denmark, and our supply chain is limited within Scandinavia. Our manufacturing facilities are carefully placed to ensure the most secure geographic, weather, political and civil conditions. We maintain safety stocks at multiple secure locations. We have full traceability of the entire supply chain.
Our Recombinant Insulin has a shelf life of 5 years. The product is dispensed into a range of HDPE plastic container, placed in individual, tamper-proof plastic bags. The tamper proofed containers are placed in approved thermo containers, inside a cardboard box and packed with sufficient dry ice to ensure that the product is kept below the recommended maximum temperature during shipment. Depending on the volume of the primary plastic containers, either a small or large shipment box is used.
Relying on Novo Nordisk’s 100 years of experience within the biopharmaceutical industry in 2023, our customers can have access to many in-house specialists who can offer their technical support, even from early product development stages. According to their needs, our customers can find a broad range of cost-effective customised solutions. Our dedicated customer support team is ready to assist with all types of requests, including documentations and shipping requests.
The target of our environmental strategy for the next 10 years of this journey is to have zero environmental impact by 2030. We collaborate proactively with suppliers to embed a circular mindset for reduced environmental impact across our value chain and switch towards circular sourcing and procurement. We aim to eliminate the environmental footprint from our operations and drive a circular transition across the company.