Benzalkonium Chloride as a good alternative to Triclosan
Triclosan (5-chloro-2-(2,4-dichlorophenoxy) phenol) is either banned or under scrutiny due to its possible effect in the disruption of hormonal development and the environment.
At equivalent antimicrobial efficacy, Benzalkonium Chloride (BKC/BAC/BAK/BZK) is a safer alternative with superior properties, including better water solubility and wider pH range of activity.
The regulatory situation
ECHA has assessed Triclosan as PBT (Persistent, bioaccumulative, and Toxic) and ED (Endocrine disrupting). In 2016, the US FDA banned it from OTC healthcare antiseptic products without premarket review.
For these reasons, you may be looking for safer alternatives.
For healthcare uses like surgical scrubs and handwashes, topical antiseptics, coating in medical devices, etc. Triclosan can be replaced by Benzalkonium Chloride (BKC/BAK/BZK).
BKC can replace Triclosan 1:1
Benzalkonium Chloride (BKC) is as effective as Triclosan in killing a wide range of microorganisms, with few variations in MIC concentrations.
The table below shows results from a test performed by the Danish Serum Institute, where colonies were suspended to a turbidity of 0.5 McFarland or 0.13 on a colorimeter, and further diluted in a broth to 1×106 CFU/ml and incubated overnight at 370C. The antimicrobial concentration range was 0.00001 to 0.01%.
Minimum Inhibitory concentration (MIC)
Mean results in % or µg/ml
RESEARCH REPORT No 182-01-04 ”Minimal Inhibitory Concentration (MIC) of Quats Benzalkonium Chloride (BKC), Cetrimonium Bromide (CTAB), Cetrimide (Tetradonium Bromide) and Triclosan against selected bacteria strains.” Statens Serum Institut, 2017.
Supported by these results, an existing product can be re-formulated with BKC using an equal concentration as Triclosan.
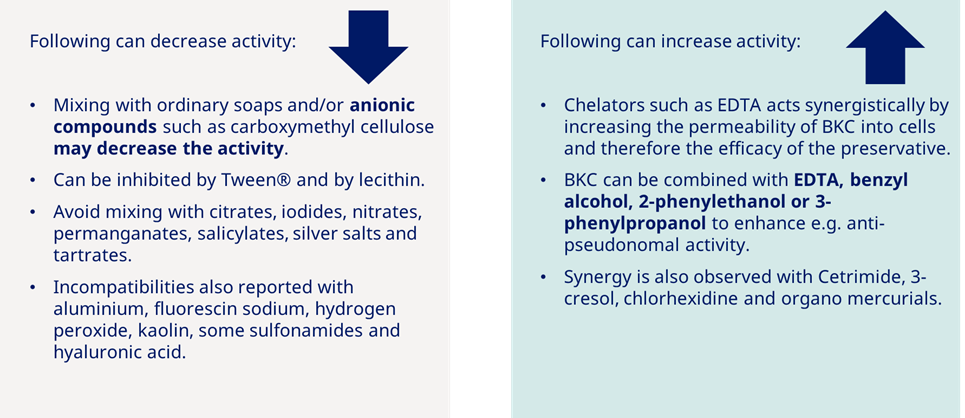
As an additional tool, here is information on BKC compatibility and incompatibility:
Benzalkonium Chloride is a safer alternative
- EU and US authorities report that there is no indication that BKC has MCR effects (mutagenic, carcinogenic, reproductive).1
- EU/ECHA: Benzalkonium Chloride is REACH registered, also as a biocide (e.g. Benzyl-C12-14-alkyldimethylammonium chlorides) with no specific safety concerns.
- EU/EMA: Approved for use in pharmaceutical formulations without restrictions.2
- Approved in both OTC and Rx pharmaceuticals, globally.
1) U.S. Environmental Protection Agency (EPA) 2006. Reregistration eligibility decision for alkyl dimethyl benzyl ammonium chloride (ADBAC) EPA739-R-06-009 U.S. Environmental Protection Agency, Washington, DC.)
2) EMA/CHMP/352187/2012 ”Report published in support of the ‘Questions and answers on benzalkonium chloride used as an excipient in medicinal products for human use”.
EMA/CHMP/495737/2013 “Questions and answers on benzalkonium chloride used as an excipient in medicinal products for human use”.)
Comparison of antimicrobials
Wondering how BKC and Triclosan compare to other antimicrobials? The table below summarizes the characteristics of several antimicrobial ingredients found on the market.
Extract from our white paper “Oral formulations – Selecting the right antiseptic for your formulation”.
Compared to Triclosan, BKC is more soluble in water and is not affected by temperature as Triclosan can be. BKC’s pH range of activity is also wider, and it offers surfactant residual activity.
Why choose Benzalkonium Chloride?
- Proven bactericide against a broad spectrum of microorganisms: Gram + and – & acid-fast bacteria, yeast, molds, enveloped viruses.
- Effective through a wide pH range (4-10/11).
- Very stable at various temperatures and pH.
- Non-volatile.
- Cost effective due to activity at low concentration.
- Strong surfactant can support active ingredient delivery and offers residual activity.
- Safe to use, non-flammable and widely available.
- Does not add color or odor to finished formulations.
- Soluble in water (Triclosan is poorly soluble in water).






