Audits
Inspection and audit-ready:
Ensuring quality at every step
Successful audits: Based on transparency and trust
Our employees are very routined in participating in audits, and we have a strong and transparent audit culture.
We take pride in our inspection and audit results, reflecting our commitment to quality, reliability, and sustainability. In total, an average of 30 customers are auditing us each year.
Audit results
2021:
Customer audits
Average rating 4.3
2022:
Customer audits
Average rating 4.5
2023:
ISO follow-up audit (9001, 14001, 45001)
Average rating 4.3
To the list of audits, we can add a document-based Sustainability audit performed by EcoVadis.
Our commitment to excellence was recognized when we achieved our first Platinum level certification at the end of 2022, a feat we repeated in 2024.
Audits and Inspections: How we maintain the “c” in front of GMP
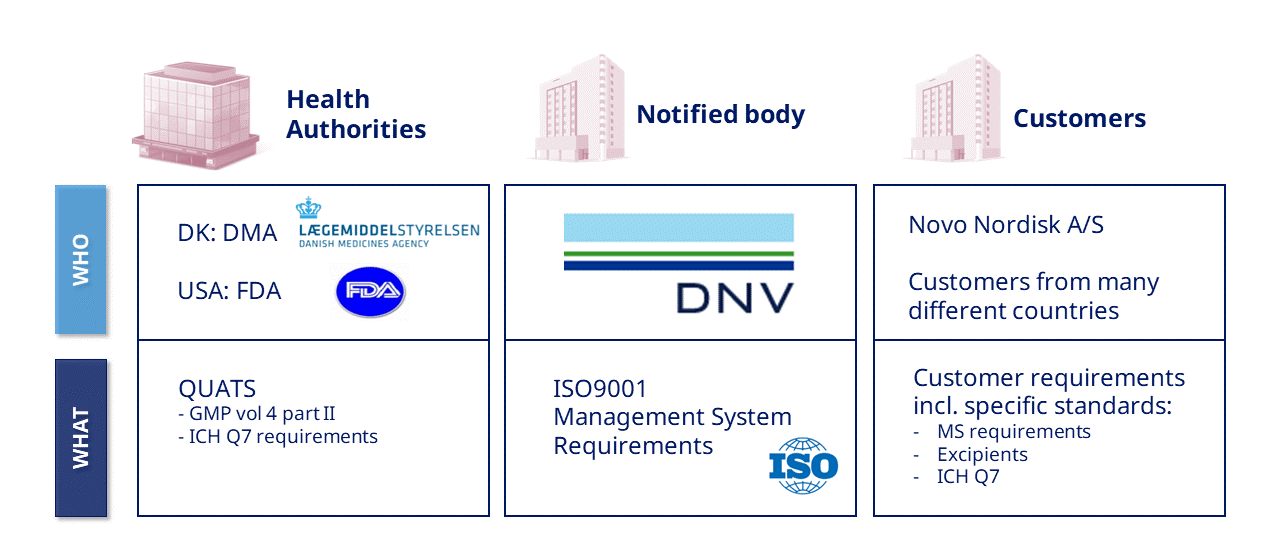
Quality Audits and Inspections
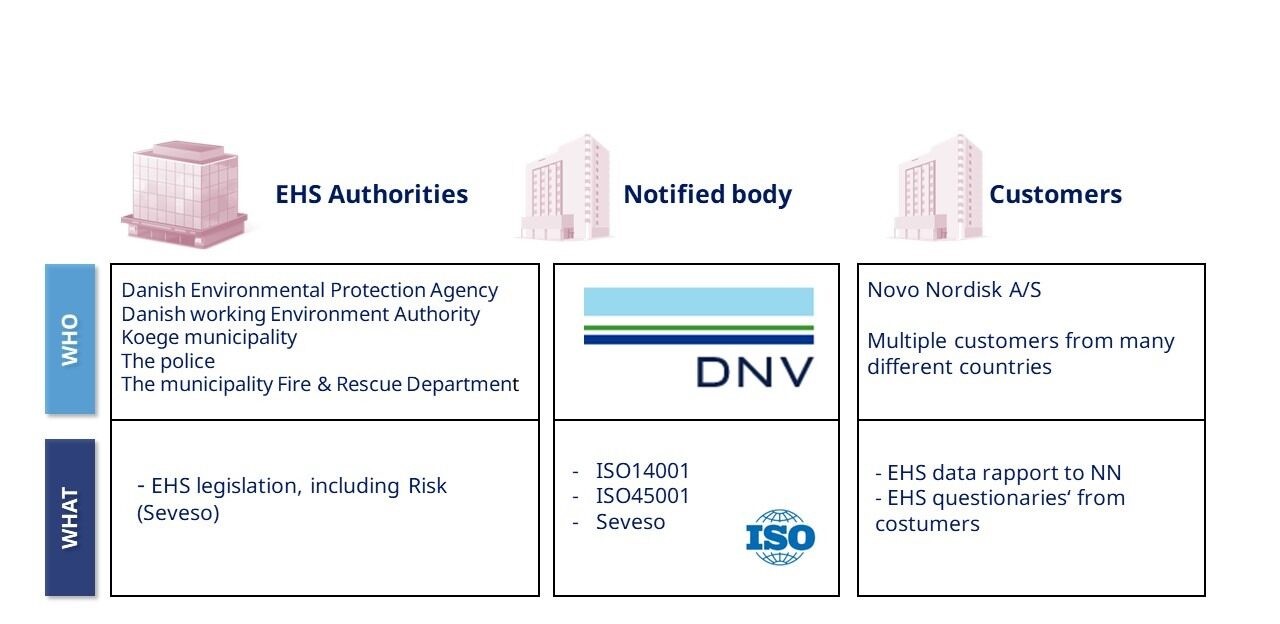
Environment, Health & Safety (EHS) Audits and Inspections
Explore more


Valuechain
Novo Nordisk Pharmatech improves biopharmaceutical manufacturing by developing and supplying innovative products used in the manufacturing of biopharmaceuticals.
