Antiseptic solutions for direct topical application will normally contain between 0.1 and 0.5% active Benzalkonium Chloride. This concentration will provide both a preservative effect for the solution and an antiseptic effect on the skin.
There are several reasons for using Benzalkonium Chloride in antiseptic products:
- Proven bactericide against a broad spectrum of microorganisms: Gram + and – & acid-fast bacteria, yeast, moulds, enveloped viruses
- Effective through a wide pH range (4-11)
- Very stable at various temperatures and pH
- Non-volatile and non-flammable
- Cost effective due to activity at low concentrations
- Strong surfactant can support active ingredient delivery and offers residual activity on the skin
- Safe to use, non-flammable and widely available
- Does not add colour or odour to finished formulations
- Soluble in both aqueous and oily phases
You can consult our white papers for the different applications here.
Product characteristics and properties
Benzalkonium Chloride is:
- Colorless and odorless in finished product formulation
- Easy to formulate in both oily and aqueous phases
- Surface active / adhesive
- Non-volatile
- Very stable
- Safe to use
Our Benzalkonium Chloride has 5 years shelf life.
Benzalkonium Chloride can be combined with other common antimicrobials, e.g., alcohol and chlorhexidine.
Mixing with ordinary soaps and/or anionic detergents may decrease the activity. As it is a cationic compound, it should not be mixed with anionic compounds, which would have a neutralizing effect.
Benzalkonium Chloride can be inhibited by Tween™ and by lecithin. Avoid mixing with citrates, iodides, nitrates, permanganates, salicylates, silver salts, and tartrates. Incompatibilities have also been reported with other substances, including aluminum, fluorescin sodium, hydrogen peroxide, kaolin, and some sulfonamides.
Benzalkonium Chloride is miscible with water or lower alcohols, such as methanol, ethanol, and propanol in all ratios. It is not miscible with benzene or ether.
Solubility decreases as the alkyl chain length increases.
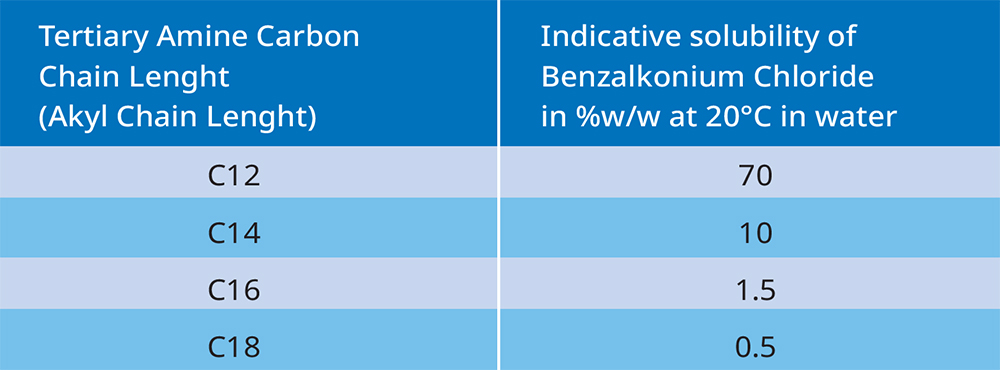
The different alkyl (fatty) chain lengths in our Benzalkonium Chloride have different properties. For example, antimicrobial activity is greater for shorter chain lengths; however, skin sensitivity decreases as the chain length increases. Shorter chain lengths are more soluble, and they also foam more.
Our production and process know-how allows us to offer Benzalkonium Chloride with a completely well-defined alkyl chain length distribution. Our standard chain length distribution is approx. 65% C12 and 35% C14, with max. 5% of C16. We also offer other chain length distributions, for example 53% C12, 30% C14, 15% C16 and 2% C18.

Benzalkonium Chloride is a bactericide and not an antibiotic. It acts on the surface of the cells and does not trigger antibiotic resistance; thus, it can be effective against antibiotic-resistant strains.
Benzalkonium Chloride is relatively non-toxic at use concentrations for pharmaceutical use and is only considered harmful in concentrated forms.
It can cause local mucosa, cornea, or skin irritation under specific conditions of prolonged use. Such irritation is reversible when ceasing to use the product.
Tertiary amines are used in the manufacture of Benzalkonium Chloride; free amine is a possible impurity and can be responsible for skin irritation. Our Benzalkonium Chloride is carefully manufactured with synthetic raw materials from qualified suppliers, and our validated processes are fully controlled to obtain the lowest possible content of impurities.
Benzalkonium Chloride is approved by authorities for pharmaceutical use. See example from the European authorities: Questions and answers on benzalkonium chloride used as an excipient in medicinal products for human use (europa.eu)
Benzalkonium Chloride is effective at all pH levels, with its effectiveness increasing when the pH increases. The higher the pH, the lower the concentration needed to obtain an antimicrobial effect.
As opposed to bacteriostatic/fungistatic compounds, which only prevent micro-organisms from dividing (growing), Benzalkonium Chloride is bactericidal, fungicidal and virucidal, meaning it will kill micro-organisms, whether they are in a growth phase or not.
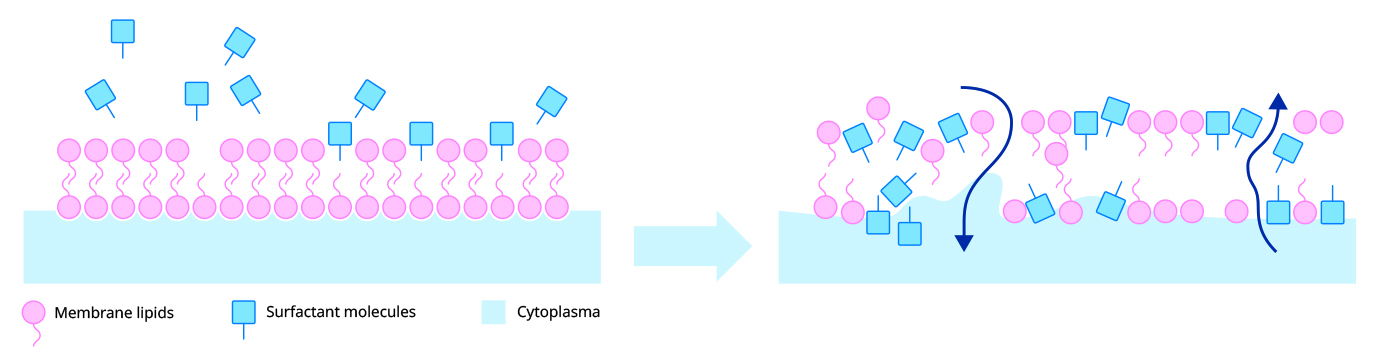
The alkyl (fatty) chains in Benzalkonium Chloride have a good affinity for bacterial membranes and cause disruption. It binds strongly to the cytoplasmic membrane evoking general membrane damage (and subsequent leakage), particularly targeting the phospholipid bilayer.
Some antibiotics under given conditions are more effective than antimicrobials. However, in general, they only work if the micro-organism is in a growth phase and thus cannot be used as an antimicrobial.
Benzalkonium Chloride has been tested against several relevant microbial strains and shown to be effective against a wide range of micro-organisms at low concentrations. In Table 1, our Benzalkonium Chloride is compared with ethanol and with a positive control containing Meropenem (a broad-spectrum antibiotic).
Benzalkonium Chloride is also effective in inactivating enveloped viruses, such as herpes, hepatitis, corona, and influenza, at very low concentrations. Several publications support its use as an effective anti-viral, for example, against SARS-CoV, M31yxo- and related viruses, type A influenza, herpes simplex, adenovirus type 5, measles, canine distemper, feline pneumonitis, meningo-pneumonitis agents, HIV-1 and more. (Please see references below)
Table 1: Minimal Inhibitory Concentrations. Mean results in % or μg/ml.*
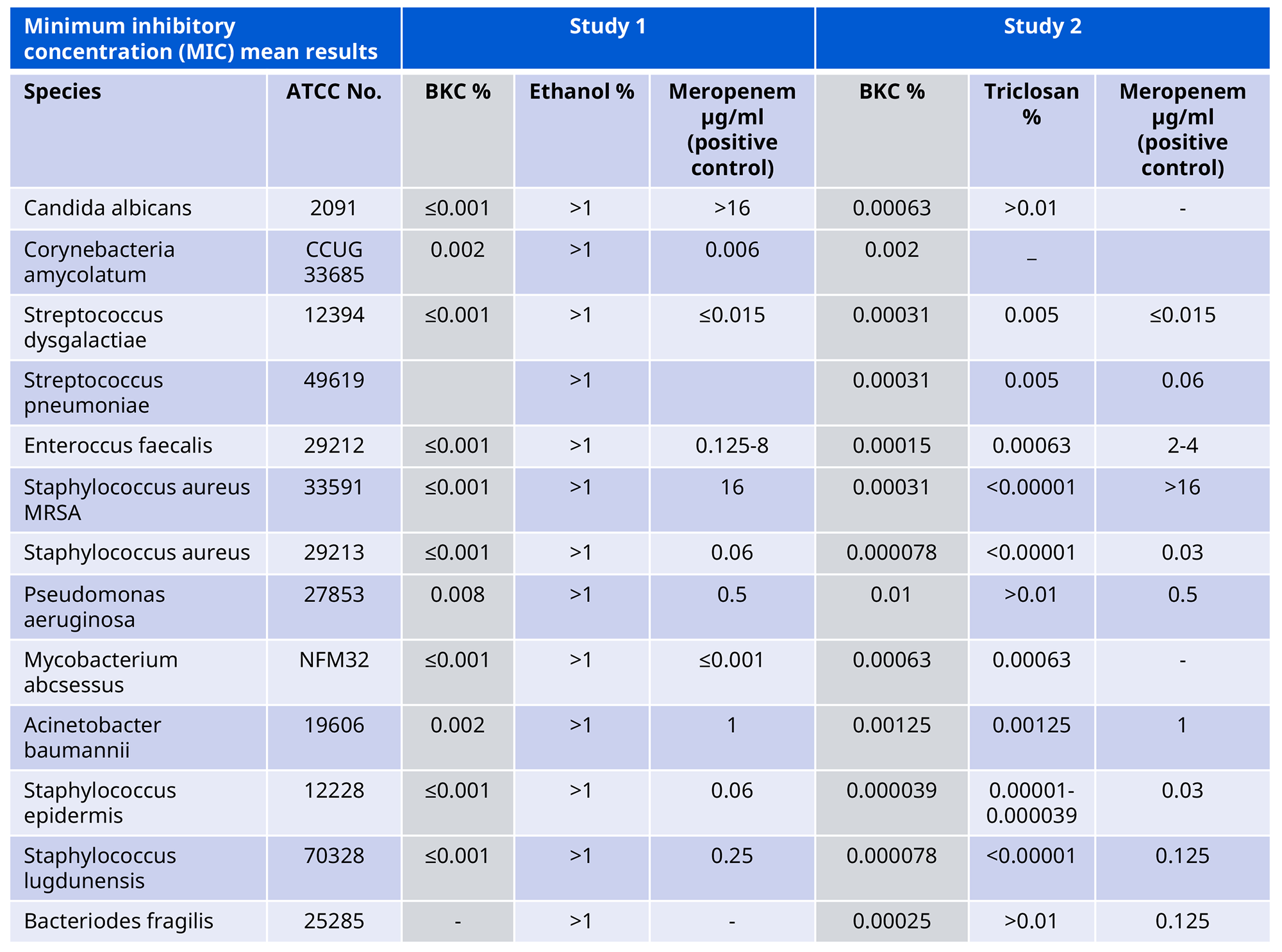
*From reports:
“Minimal Inhibitory Concentration (MIC) of Quats Benzalkonium Chloride (BKC), Cetrimonium Bromide (CTAB) and Cetrimide (Tetradonium Bromide) against selected bacteria strains.” Statens Serum Institute, Denmark, 2016.
”Minimal Inhibitory Concentration (MIC) of Quats Benzalkonium Chloride (BKC), Cetrimonium Bromide (CTAB), Cetrimide (Tetradonium Bromide) and Triclosan against selected bacteria strains.” Statens Serum Institute, Denmark, 2017.
References
- Saknimit M, Inatsuki I, Sugiyama Y, Yagami K. (1988). Virucidal efficacy of physico-chemical treatments against coronaviruses and parvoviruses of laboratory animals. Jikken Dobutsu. 37:341-5.
- Centers for Disease Control and Prevention. (2008) Guideline for Disinfection and Sterilization in Healthcare Facilities, U.S. Centers for Disease Control and Prevention.
- Dellanno C1, Vega Q, Boesenberg D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. American Journal of Infection Control.2009 Oct;37(8):649-52.
- A. Armstrong and E.J. Froelich. Inactivation of Viruses by Benzalkonium Chloride. Applied Microbiology Vol. 12. No. 2,132-137 March 1964.
- Manfred H.Wolff, Syed A. Sattar, Olusola Adegbunrin and Jason Tetro. Environmental survival and microbicide inactivation of coronaviruses. Coronaviruses with Special Emphasis on First Insights Concerning SARS 201 ed. by A. Schmidt, M.H. Wolff and O. Weber, 2005, p.201-212.
- Mark A. Wainberg, Bonnie Spira, Gilles Bleau and Rejean Thomas. Inactivation of Human Immunodeficiency Virus Type 1 in Tissue Culture Fluid and in Genital Secretions by the Spermicide Benzalkonium Chloride. Journal of Clinical Microbiology, Jan. 1990. p. 156-158
Looking for a replacement for Triclosan? Click here for a product comparison with Benzalkonium Chloride.